Unit Cells :
In this topic we would be studying certain properties of a solid which depend only on the constituents of the solid and the pattern of arrangement of these constituents. The smallest amount of the solid whose properties resemble the properties of the entire solid irrespective of the amount taken is called a unit cell. It is the smallest repeating unit of the solid. Any amount of the solid can be constructed by simply putting as many unit cells as required.
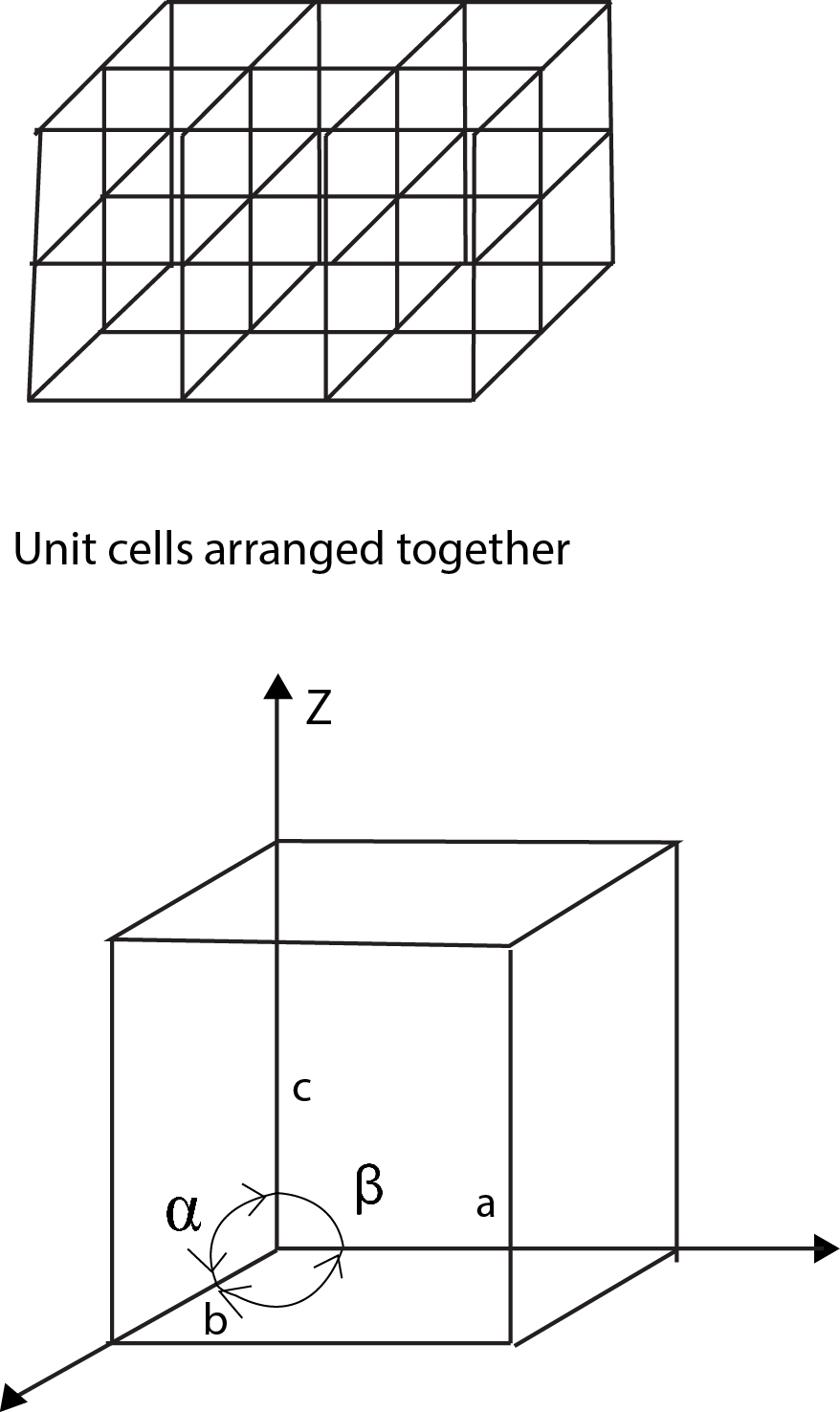
In a `3`-dimensional space lattice, to specify a unit cell we need the values of three vectors which gives three distances along the three axes and three angles as shown in figure
In a `3`-dimensional space lattice, to specify a unit cell we need the values of three vectors which gives three distances along the three axes and three angles as shown in figure