Octahedral and Tetrahedral Void :
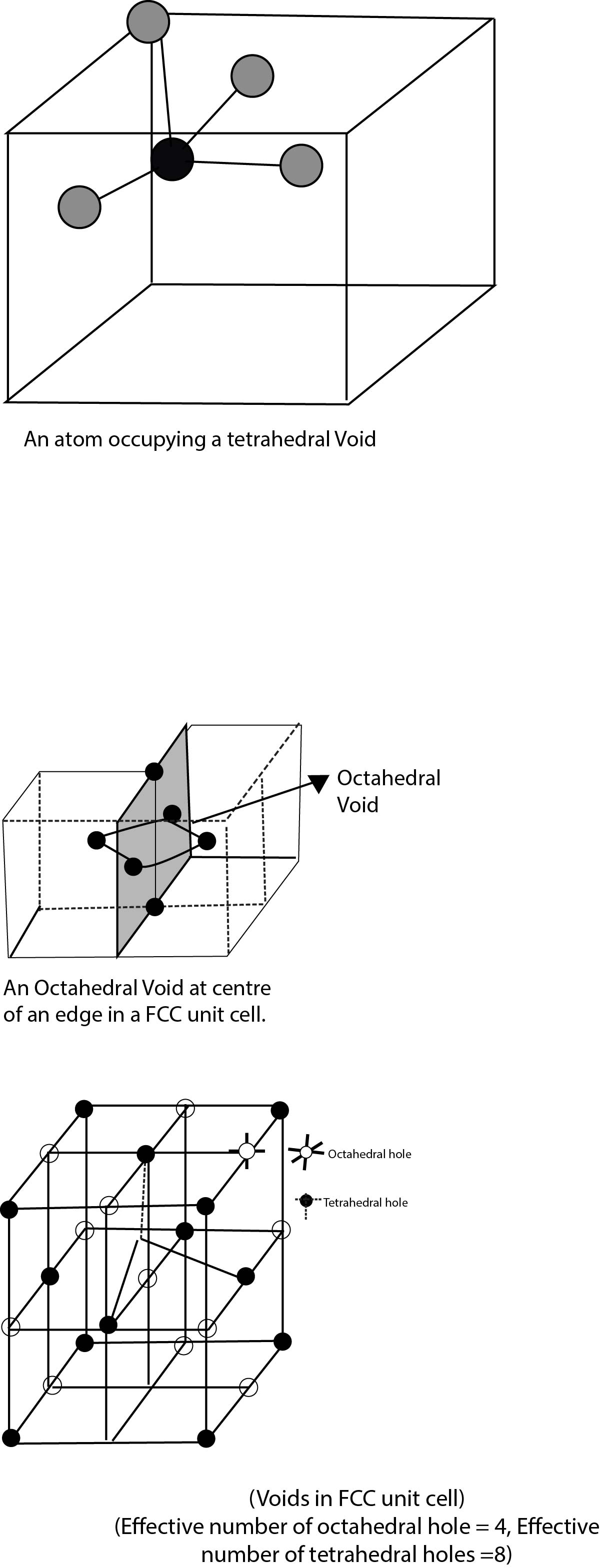
The close packing system involves two kinds of voids - tetrahedral voids and octahedral voids. The former has four spheres adjacent to it while the latter has six spheres adjacent to it. These voids are only found in either fcc or Hexagonal Primitive unit cells.
A tetrahedral void is formed when an atom fits into the depression formed by three other closest packed atoms (forming an equilateral triangle). All the four atoms are in contact with each other. If we join the centre of all four atoms, we get a tetrahedron. The centre of tetrahedron represents a tetrahedral void.
An octahedral void is formed when three closest packed atoms of one layer (forming an equilateral triangle) is placed over three closest packed atoms of the second layer, their positions being inverted with respect to each other. Each atom touches four other atoms, except the atom diagonally opposite to it.
A tetrahedral void is formed when an atom fits into the depression formed by three other closest packed atoms (forming an equilateral triangle). All the four atoms are in contact with each other. If we join the centre of all four atoms, we get a tetrahedron. The centre of tetrahedron represents a tetrahedral void.
An octahedral void is formed when three closest packed atoms of one layer (forming an equilateral triangle) is placed over three closest packed atoms of the second layer, their positions being inverted with respect to each other. Each atom touches four other atoms, except the atom diagonally opposite to it.