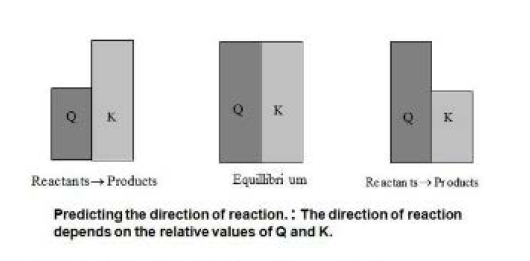
Let us consider a reaction of the type,
`A_(g) +B_(g) ⇋ C_(g) +D_(g)`
The double headed arrow signifies that the reaction occurs in both the directions in measurable extent. You start with pure `A` & `B` which are the reactants, in a closed system. Initially `A` and `B` reacts very fast to give `C` and `D`. Now as soon as `C` and `D` are formed they also started giving back `A` and `B`. But `A` and `B` being present in greater quantity forces the reaction to occur in forward direction much faster than the backward one. So what is the net result? It's nothing but the net formation of `C` & `D`. In this way as the time passes, `A` & `B` decreases and hence their strength, on the other hand `C` & `D` increases and so is their strength. So ultimately a time comes when the forward reaction is balanced by backward reaction. This state is the equilibrium.
Now, before deriving the different related expression let us know what is Law of Mass Action. It states that,
''The rate of a reaction is directly proportional to the product of concentrations of reactants with the stoichiometric co-efficient being raised to the power"
e.g. for the reaction.
`aA_( g) + bB_(g) ⇋ cC_(g) + dD_(g)`
for the forward reaction,
`R_f prop [A]^a[B]^b`
Where `R_f` denotes rate of forward reaction.
`[A]` denotes concentration of `A`
`'a'`, `'b'` are the powers which are the coefficients of `A` & `B` respectively.
If `k_f` be the rate constant for forward reaction the expression can be rewritten as
`R_f = k_f[A]^a [B]^b ... (1)`
Similarly, for the backward reaction :
`R_b = k_b[C]^c[D]^d...............(2)`
At equilibrium :
Rate of forward reaction = Rate of backward reaction
From equation (1) and (2)
`R_f = R_b`
or `k_f [A]^a [B]^b = k_b [C]^c [D]^d`
Rearranging we get, `k_f/k_b = ([C]^c[D]^d)/([A]^a[D]^d)`
Now, the expression on the L.H.S. is a constant. So, it means the expression on the R.H.S. has to be a constant. In fact it is so for a particular reaction. But obviously at a given temperature. Effect of temperature we will study later. This constant is named as equilibrium constant and is denoted by `K`.
This implies that no matter what we start with (i.e., `A` & `B` or `C` & `D` or `A+B+C` or `A+B+D` or `A+C+D` or `B+C+D` or `A+B+C+D`) and how much of these we start with, the ratio of `([C]^c[D]^d)/([A]^a[B]^b)` is a fixed quantity at a given temperature when the reaction reaches equilibrium. That is, if we assume that this reaction has `K = 4` then no matter what we take initially and irrespective of how much we take, once equilibrium is reached the ratio of `([C]^c[D]^d)/([A]^a[B]^b)` will always be equal to `4`.
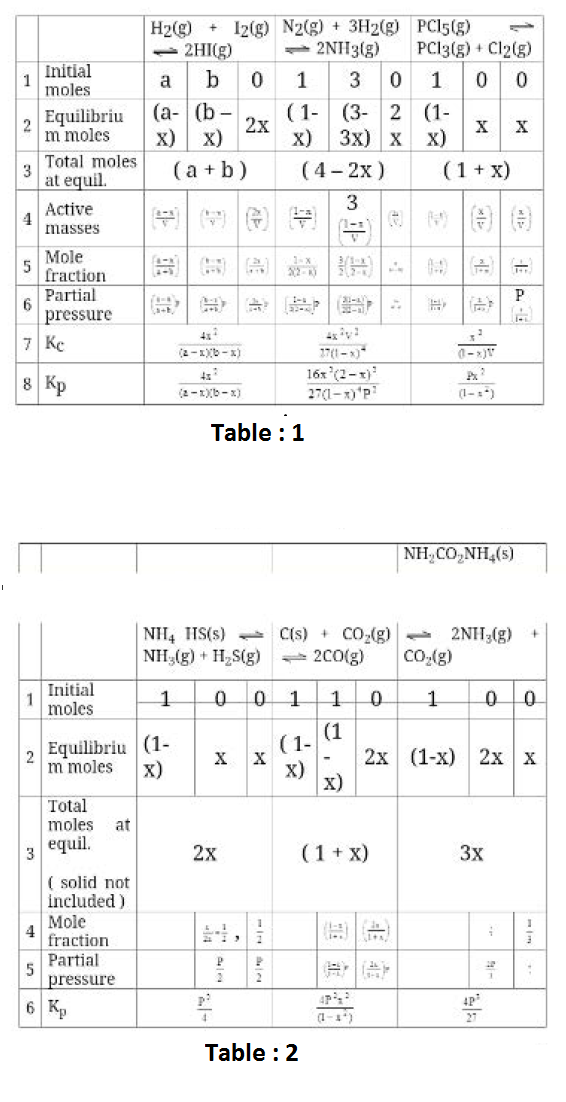
Now let us consider the following reaction
`A(s) + B(g) ⇋ C(s) + D(g)`
Its equilibrium constant, `K` would be; `K = ([C][D])/([A][B])`
Concentration of `D` is the number of moles of `D` per unit volume of the container (we can assume that the gas occupies the entire container). The concentration of `A` is the number of moles of `A` per unit volume of `A`. The concentration of all solids and pure liquids is a constant. This is because if initially we take `w` gm of `A`, then the moles of `A` are `w//M`. The volume of `A` is `w//d` where `d` is the density of `A`. Therefore, the initial concentration of `A` is `(w/M)//(w/d) = d/M` We can see that at equilibrium also the concentration of `A` remains as `d//M` (`d` and `M` are constants). In fact even if `A` were a pure liquid, its concentration would have remained constant.
Therefore, we bring all the constant terms on one side and we get
`(K[A])/[C] = [D]/[B]`
This ratio which is a constant and which involves only those concentration terms which are variables is called `K_c` the equilibrium constant in terms of concentration.
`K _c = [D]/[B] = k[A]/[C]`
Now, let us consider the reaction,
`A(g) + 2B(g) ⇋ 3C (g) + 4D (g)`
`K_c = ([C]^3[D]^4)/([A][B]^2)`
We know that concentration of a gas can be expressed as `P//RT` as shown below :
`PV =nRT`
`P/(RT) =n/V =` number of moles per litre= concentration
`[C]= P_C/(RT)`; `[D] = P_D/(RT)`; `[A]= P_A/(RT)` and `[B] = P_B/(RT)`
Where,
`P_C =` partial pressure of `C`
`P_D =` partial pressure of `D`
`P_A =` partial pressure of `A`
`P_B =` partial pressure of `B`
`K_C = ((P_C/(RT))^3(P_D/(RT))^4)/((P_A/(RT))(P_B/(RT))^2)`
`(P_C^3P_D^4)/(P_AP_B^2) = K_c(RT)^((3+4) -(1+2))`.
Since `K_c` is a constant and `RT` is also a constant, so the right hand side of the above expression is also a constant. This is called `K_p`, the equilibrium constant in terms of the partial pressure.
`(P_C^3P_D^4)/(P_AP_B^2) = K_p`
Therefore, for this particular equilibrium, the ratio of partial pressures is also a constant.
In general, the relation between `K_p` and `K_c` is `K_p = K_c (RT)^(Deltan)`
Where `Deltan =` number of moles of gaseous products - number of moles of gaseous reactants.
Now `Delta n` can have three possibilities.
`Delta n < 0`, `Delta n = 0`, `Delta n > 0`
Accordingly we can predict, out of `K_p` and `K_c` which one will be higher or lower. At `Delta n = 0` both `K_p` & `K_c` are the same.
Now let us assume that `A` is a solid or pure liquid. The changes now would be that `K_c` would look like this, `K_c = ([C]^3[D]^4)/[B]^2` and following the above given sequence of derivation, `K_p` would look like this
`K_p = (P_C^3P_D^4)/P_B^2`
Next we assume that `A` was a solute present in a solution then `K_c` would remain the same i.e, `K_c = ([C]^3[D]^4)/([A][B]^2)`. Now if we try to express the concentrations in terms of partial pressures, we would fail to do that for `A`. It is not possible to express the concentration of a solution in terms of its pressure or vapour pressure and constants.
Therefore `[A]` remains as such
`K_c = ((P_C/(RT))^2(P_C/(RT))^4)/([A](P_B/(RT))^2)` `(P_C^2P_D^4)/([A]P_B^2) = K_c(RT)^((3+4)-(2))`
The R.H.S. of the above expression is a constant which implies that the L.H.S is also a constant. This new expression cannot be called as either `K_c` or `K_p` since it contains both concentration terms and pressure terms. We call it `K_(pc)`. We can also see that if we take `[A]` to the R.H.S. the L.H.S. contains only pressure terms, but then it is not a constant since `[A]` is not a constant.
Therefore, we can conclude that for `K_p` to exist for a reaction it must fulfill two conditions :- (i) it must have at least one gas either in the reactants or in the products and (ii) it must not have any component in solution phase.
Let us consider a reaction of the type,
`A_(g) +B_(g) ⇋ C_(g) +D_(g)`
The double headed arrow signifies that the reaction occurs in both the directions in measurable extent. You start with pure `A` & `B` which are the reactants, in a closed system. Initially `A` and `B` reacts very fast to give `C` and `D`. Now as soon as `C` and `D` are formed they also started giving back `A` and `B`. But `A` and `B` being present in greater quantity forces the reaction to occur in forward direction much faster than the backward one. So what is the net result? It's nothing but the net formation of `C` & `D`. In this way as the time passes, `A` & `B` decreases and hence their strength, on the other hand `C` & `D` increases and so is their strength. So ultimately a time comes when the forward reaction is balanced by backward reaction. This state is the equilibrium.
Now, before deriving the different related expression let us know what is Law of Mass Action. It states that,
''The rate of a reaction is directly proportional to the product of concentrations of reactants with the stoichiometric co-efficient being raised to the power"
e.g. for the reaction.
`aA_( g) + bB_(g) ⇋ cC_(g) + dD_(g)`
for the forward reaction,
`R_f prop [A]^a[B]^b`
Where `R_f` denotes rate of forward reaction.
`[A]` denotes concentration of `A`
`'a'`, `'b'` are the powers which are the coefficients of `A` & `B` respectively.
If `k_f` be the rate constant for forward reaction the expression can be rewritten as
`R_f = k_f[A]^a [B]^b ... (1)`
Similarly, for the backward reaction :
`R_b = k_b[C]^c[D]^d...............(2)`
At equilibrium :
Rate of forward reaction = Rate of backward reaction
From equation (1) and (2)
`R_f = R_b`
or `k_f [A]^a [B]^b = k_b [C]^c [D]^d`
Rearranging we get, `k_f/k_b = ([C]^c[D]^d)/([A]^a[D]^d)`
Now, the expression on the L.H.S. is a constant. So, it means the expression on the R.H.S. has to be a constant. In fact it is so for a particular reaction. But obviously at a given temperature. Effect of temperature we will study later. This constant is named as equilibrium constant and is denoted by `K`.
This implies that no matter what we start with (i.e., `A` & `B` or `C` & `D` or `A+B+C` or `A+B+D` or `A+C+D` or `B+C+D` or `A+B+C+D`) and how much of these we start with, the ratio of `([C]^c[D]^d)/([A]^a[B]^b)` is a fixed quantity at a given temperature when the reaction reaches equilibrium. That is, if we assume that this reaction has `K = 4` then no matter what we take initially and irrespective of how much we take, once equilibrium is reached the ratio of `([C]^c[D]^d)/([A]^a[B]^b)` will always be equal to `4`.
Now let us consider the following reaction
`A(s) + B(g) ⇋ C(s) + D(g)`
Its equilibrium constant, `K` would be; `K = ([C][D])/([A][B])`
Concentration of `D` is the number of moles of `D` per unit volume of the container (we can assume that the gas occupies the entire container). The concentration of `A` is the number of moles of `A` per unit volume of `A`. The concentration of all solids and pure liquids is a constant. This is because if initially we take `w` gm of `A`, then the moles of `A` are `w//M`. The volume of `A` is `w//d` where `d` is the density of `A`. Therefore, the initial concentration of `A` is `(w/M)//(w/d) = d/M` We can see that at equilibrium also the concentration of `A` remains as `d//M` (`d` and `M` are constants). In fact even if `A` were a pure liquid, its concentration would have remained constant.
Therefore, we bring all the constant terms on one side and we get
`(K[A])/[C] = [D]/[B]`
This ratio which is a constant and which involves only those concentration terms which are variables is called `K_c` the equilibrium constant in terms of concentration.
`K _c = [D]/[B] = k[A]/[C]`
Now, let us consider the reaction,
`A(g) + 2B(g) ⇋ 3C (g) + 4D (g)`
`K_c = ([C]^3[D]^4)/([A][B]^2)`
We know that concentration of a gas can be expressed as `P//RT` as shown below :
`PV =nRT`
`P/(RT) =n/V =` number of moles per litre= concentration
`[C]= P_C/(RT)`; `[D] = P_D/(RT)`; `[A]= P_A/(RT)` and `[B] = P_B/(RT)`
Where,
`P_C =` partial pressure of `C`
`P_D =` partial pressure of `D`
`P_A =` partial pressure of `A`
`P_B =` partial pressure of `B`
`K_C = ((P_C/(RT))^3(P_D/(RT))^4)/((P_A/(RT))(P_B/(RT))^2)`
`(P_C^3P_D^4)/(P_AP_B^2) = K_c(RT)^((3+4) -(1+2))`.
Since `K_c` is a constant and `RT` is also a constant, so the right hand side of the above expression is also a constant. This is called `K_p`, the equilibrium constant in terms of the partial pressure.
`(P_C^3P_D^4)/(P_AP_B^2) = K_p`
Therefore, for this particular equilibrium, the ratio of partial pressures is also a constant.
In general, the relation between `K_p` and `K_c` is `K_p = K_c (RT)^(Deltan)`
Where `Deltan =` number of moles of gaseous products - number of moles of gaseous reactants.
Now `Delta n` can have three possibilities.
`Delta n < 0`, `Delta n = 0`, `Delta n > 0`
Accordingly we can predict, out of `K_p` and `K_c` which one will be higher or lower. At `Delta n = 0` both `K_p` & `K_c` are the same.
Now let us assume that `A` is a solid or pure liquid. The changes now would be that `K_c` would look like this, `K_c = ([C]^3[D]^4)/[B]^2` and following the above given sequence of derivation, `K_p` would look like this
`K_p = (P_C^3P_D^4)/P_B^2`
Next we assume that `A` was a solute present in a solution then `K_c` would remain the same i.e, `K_c = ([C]^3[D]^4)/([A][B]^2)`. Now if we try to express the concentrations in terms of partial pressures, we would fail to do that for `A`. It is not possible to express the concentration of a solution in terms of its pressure or vapour pressure and constants.
Therefore `[A]` remains as such
`K_c = ((P_C/(RT))^2(P_C/(RT))^4)/([A](P_B/(RT))^2)` `(P_C^2P_D^4)/([A]P_B^2) = K_c(RT)^((3+4)-(2))`
The R.H.S. of the above expression is a constant which implies that the L.H.S is also a constant. This new expression cannot be called as either `K_c` or `K_p` since it contains both concentration terms and pressure terms. We call it `K_(pc)`. We can also see that if we take `[A]` to the R.H.S. the L.H.S. contains only pressure terms, but then it is not a constant since `[A]` is not a constant.
Therefore, we can conclude that for `K_p` to exist for a reaction it must fulfill two conditions :- (i) it must have at least one gas either in the reactants or in the products and (ii) it must not have any component in solution phase.