`Br``ddot o`nsted - Lowry Concept (The Proton-donor-Acceptor Concept) :
According to Bronsted-Lowry theory, an acid is a substance that is capable of donating a hydrogen ion `H^(+)` and bases are substances capable of accepting a hydrogen ion, `H^(+)`. In short, acids are proton donors and bases are proton acceptors.
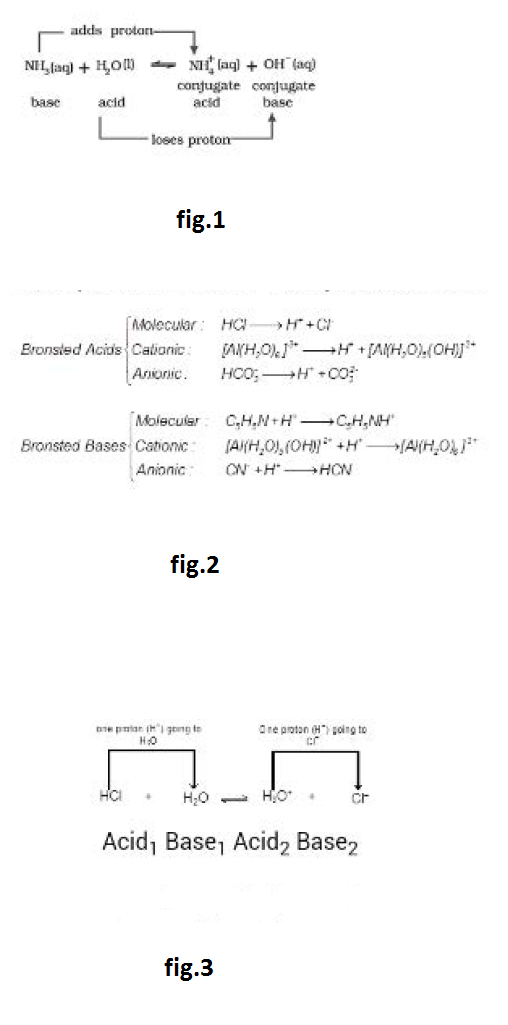
For eg., the dissolution of ammonia in water can be represented as See fig.1.
In this reaction, water molecule acts as proton donor and ammonia molecule acts as proton acceptor and are thus, called Lowry-Bronsted acid and base, respectively. For the backward reaction, `NH_4^(+)` donates `H^+`, hence it is an acid; `OH^-` accepts `H^+`, hence it is base.
Examples of Bronsted - Lowry Acids & Bases- See fig.2.
`text(Conjugate Acid-Base Pair Concept)` -
An acid-base pair that differs only by one proton is called a conjugate acid-base pair
`text(Consider a reaction)` See fig.3.
In this reaction `HCl` donates a proton to `H_2O` and is, therefore an acid. Water, on the other hand, accepts a proton from `HCl`, and is, therefore, a base. In the reverse reaction, the `H_3O^(+)` ion donates a proton to `Cl^(-)` ion, hence `H_3O^(+)` ion is an acid. `Cl^(-)` ion is a base.
`HCl underset(+H^(+)) overset(-H^(+))⇋ Cl^(-)` and `H_3O^+ underset(+H^(+))overset(-H^(+)) ⇋ H_2O`. Acid base pairs such as which can be formed from each other mutually by the gain or loss of a proton are called conjugate acid - base pairs.
If in the above reaction, the acid `HCl` is an acid and `Cl^-` is its conjugate base.
Similarly, `H_2O` is a base and `H_3O^(+)` is its conjugate acid.
`text(Note-)`
- To get conjugate acid of a given species add `H^(+)` to it. e.g. conjugate acid of `N_2H_4` is `N_2H_5^(+)`.
- To get conjugate base of any species subtract `H^(+)` from it. e.g. Conjugate base of `NH_3` is `NH_2^-`.
- Stronger a Bronsted acid is, weaker is its conjugate base.
- Stronger a Bronsted base is, weaker is its conjugate acid.
More examples -
`text(Acid Conjugate base)`
(i) `HCl quad quad Cl^(-)`
(ii) `H_2SO_4 quad quad HSO_4^(-)`
(iii) `HSO_ 4^(-) quad quad SO_4^(2-)`
(iv) `H_2O quad quad OH^(-)`
`text(Base Conjugate acid)`
(i) `NH_3 quad quad NH_4^(+)`
(ii) `H_2O quad quad H_3O^(+)`
(iii) `RNH_2 quad quad RNH_3^(+)`
`text(Amphiprotic substances)`
Substance that act as an acid as well as a base is called as amphiprotic.
Water can act as an acid in the presence of bases stronger than itself such as `NH_3`, amine, `C_2H_5O^(-)`, `OH^(-)` and `CO_3^(2-)` ions. Water can act as a base in the presence of acids stronger than itself such as `HClO_4`, `HCl`, `CH_3COOH` and phenol.
In fact the amphiprotic nature of `H_2O` is well illustrated in the extremely slight dissociation or self-ionisation :
`oversettext(Weaker acid)(H_2O) +oversettext(Weaker base)(H_2O) ⇋ oversettext(Stronger acid)(H_3O^(+)) + oversettext(Stronger base)(OH^(-))` `(k_w = 1.0 xx 10^(-14))`
For eg., the dissolution of ammonia in water can be represented as See fig.1.
In this reaction, water molecule acts as proton donor and ammonia molecule acts as proton acceptor and are thus, called Lowry-Bronsted acid and base, respectively. For the backward reaction, `NH_4^(+)` donates `H^+`, hence it is an acid; `OH^-` accepts `H^+`, hence it is base.
Examples of Bronsted - Lowry Acids & Bases- See fig.2.
`text(Conjugate Acid-Base Pair Concept)` -
An acid-base pair that differs only by one proton is called a conjugate acid-base pair
`text(Consider a reaction)` See fig.3.
In this reaction `HCl` donates a proton to `H_2O` and is, therefore an acid. Water, on the other hand, accepts a proton from `HCl`, and is, therefore, a base. In the reverse reaction, the `H_3O^(+)` ion donates a proton to `Cl^(-)` ion, hence `H_3O^(+)` ion is an acid. `Cl^(-)` ion is a base.
`HCl underset(+H^(+)) overset(-H^(+))⇋ Cl^(-)` and `H_3O^+ underset(+H^(+))overset(-H^(+)) ⇋ H_2O`. Acid base pairs such as which can be formed from each other mutually by the gain or loss of a proton are called conjugate acid - base pairs.
If in the above reaction, the acid `HCl` is an acid and `Cl^-` is its conjugate base.
Similarly, `H_2O` is a base and `H_3O^(+)` is its conjugate acid.
`text(Note-)`
- To get conjugate acid of a given species add `H^(+)` to it. e.g. conjugate acid of `N_2H_4` is `N_2H_5^(+)`.
- To get conjugate base of any species subtract `H^(+)` from it. e.g. Conjugate base of `NH_3` is `NH_2^-`.
- Stronger a Bronsted acid is, weaker is its conjugate base.
- Stronger a Bronsted base is, weaker is its conjugate acid.
More examples -
`text(Acid Conjugate base)`
(i) `HCl quad quad Cl^(-)`
(ii) `H_2SO_4 quad quad HSO_4^(-)`
(iii) `HSO_ 4^(-) quad quad SO_4^(2-)`
(iv) `H_2O quad quad OH^(-)`
`text(Base Conjugate acid)`
(i) `NH_3 quad quad NH_4^(+)`
(ii) `H_2O quad quad H_3O^(+)`
(iii) `RNH_2 quad quad RNH_3^(+)`
`text(Amphiprotic substances)`
Substance that act as an acid as well as a base is called as amphiprotic.
Water can act as an acid in the presence of bases stronger than itself such as `NH_3`, amine, `C_2H_5O^(-)`, `OH^(-)` and `CO_3^(2-)` ions. Water can act as a base in the presence of acids stronger than itself such as `HClO_4`, `HCl`, `CH_3COOH` and phenol.
In fact the amphiprotic nature of `H_2O` is well illustrated in the extremely slight dissociation or self-ionisation :
`oversettext(Weaker acid)(H_2O) +oversettext(Weaker base)(H_2O) ⇋ oversettext(Stronger acid)(H_3O^(+)) + oversettext(Stronger base)(OH^(-))` `(k_w = 1.0 xx 10^(-14))`
