Basic Character of Amines :
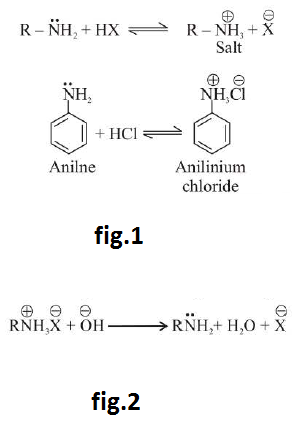
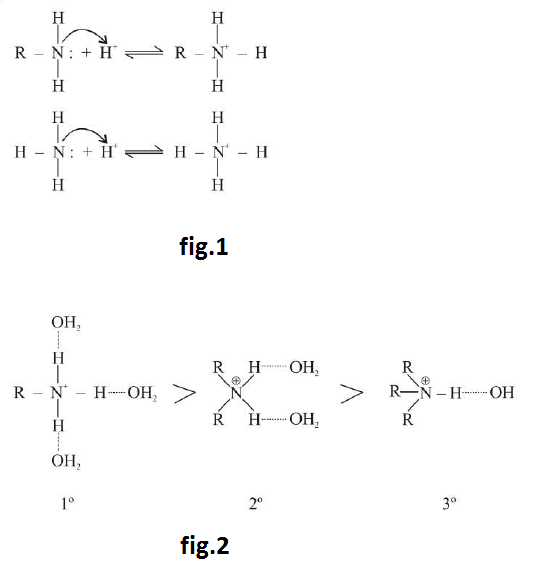
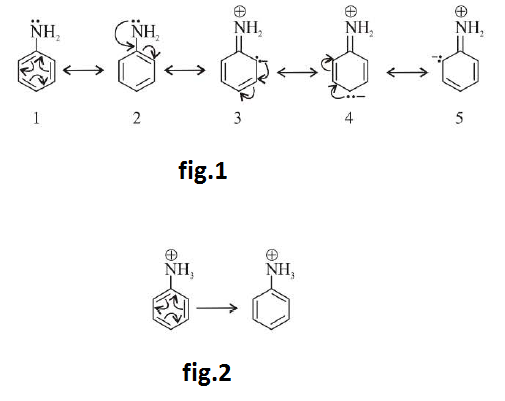
Amines are relatively weak bases. They are stronger bases than water but are far weaker bases than hydroxides ions (`OH^-`), alkoxide ions (`RO^-`) and alkanide (`R:^-`) anions. These react with acids to form salts. See fig.1.
On reaction with a base such as `NaOH`, amine salt regenerate parent amine. See fig.2.
On reaction with a base such as `NaOH`, amine salt regenerate parent amine. See fig.2.