Free Radicals
`text(Structure and geometry :)` A free redical is a species which has one or more unpaired electrons . In the species, where all electrons are paired the total magnetic moment is zero. In redicals, however, there are one or more unpaired electrons,there is a net magnetic moment and the redical as a result are paramagnetic .Free redical are usually detected by electron spin resonance , which is also terms electron para magnetic resonance .
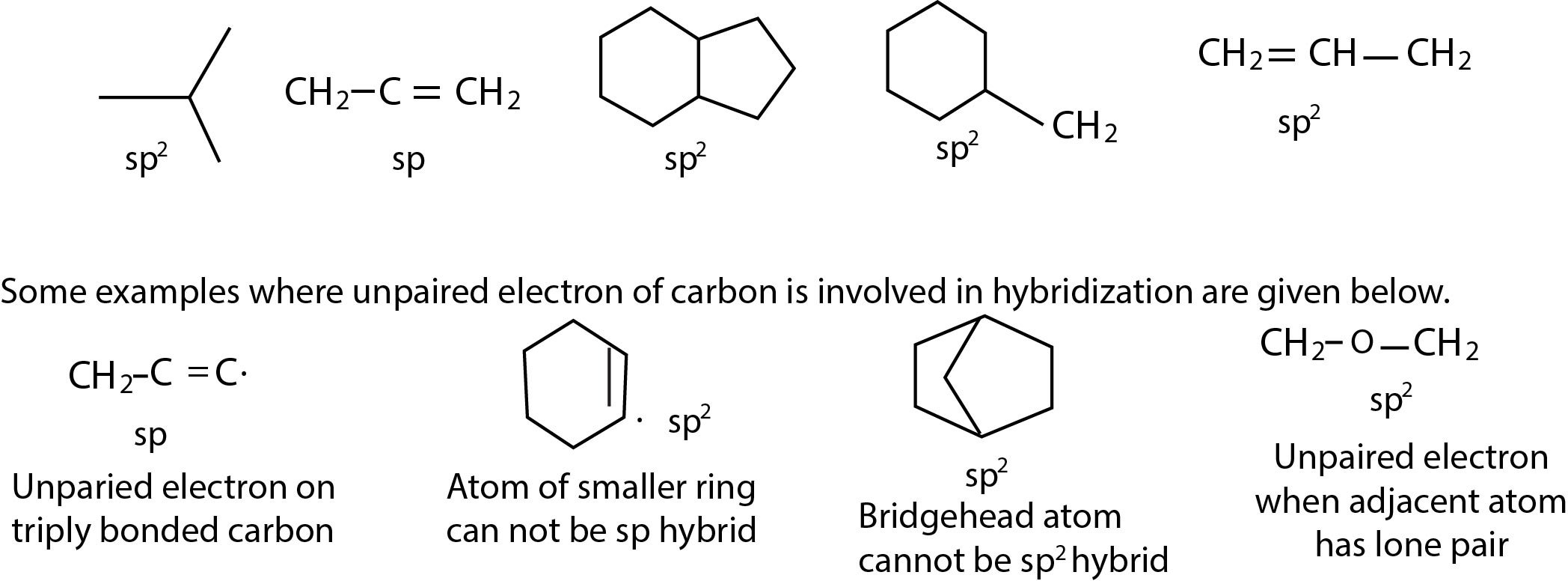
Simply alkyl radicals have a planer (trogonal) structure , i ., e these have `sp^2` bonding with the odd electronin a p orbital . the pyramidasl structure is another possibility when the bonding may be `sp^3` and the odd electron is an `sp^3` orbotal . the planer structure is kjeeping with loss of activity when a free redical is gene rated at a chiral centre . Thus a planer redical will attacked at either face after its formation with equal probability to give enantiomers generated as bridge . This show that pyramidal geometry for redical is also possible and that free reducals need to be planer .
Simply alkyl radicals have a planer (trogonal) structure , i ., e these have `sp^2` bonding with the odd electronin a p orbital . the pyramidasl structure is another possibility when the bonding may be `sp^3` and the odd electron is an `sp^3` orbotal . the planer structure is kjeeping with loss of activity when a free redical is gene rated at a chiral centre . Thus a planer redical will attacked at either face after its formation with equal probability to give enantiomers generated as bridge . This show that pyramidal geometry for redical is also possible and that free reducals need to be planer .