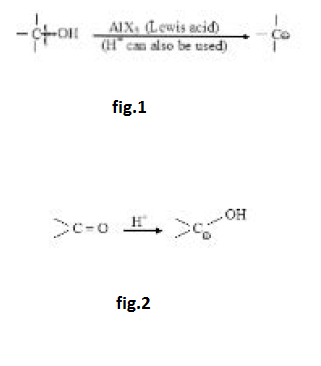
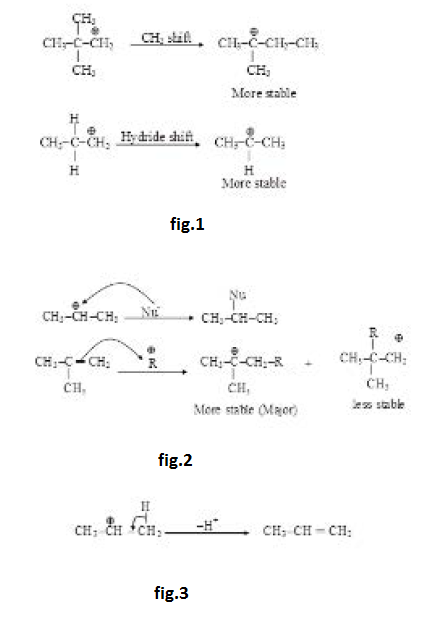
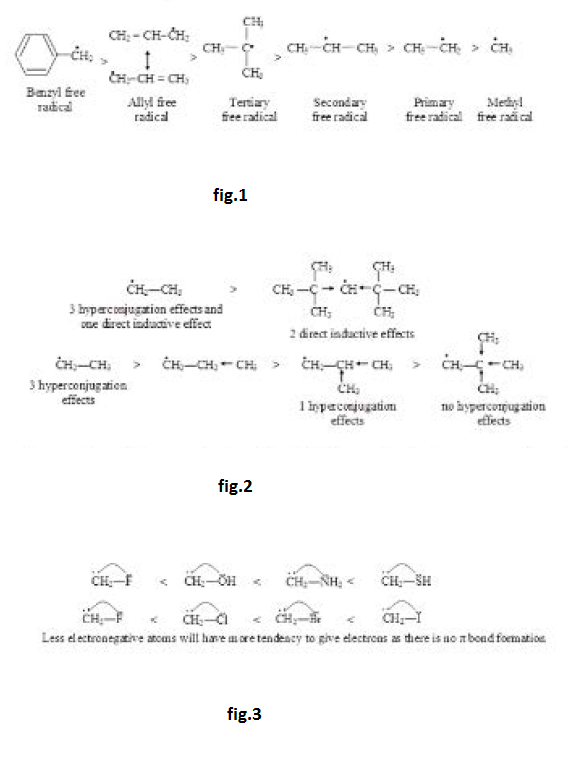
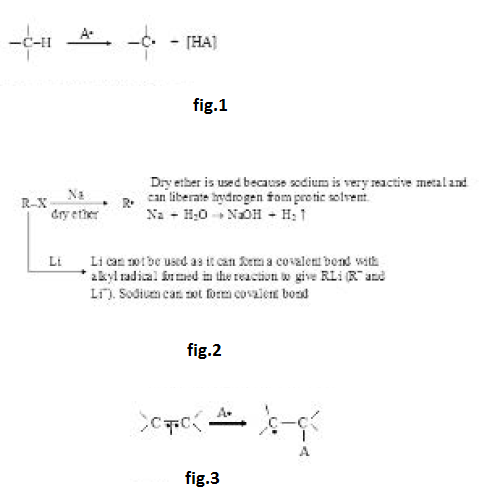
Stability of Carbocation :

Stability of carbocation depends on factors affecting electron density. Any factor decreasing the positive charge will increase the stability. Effect of resonance is generally much more, direct inductive effect dominates over hyperconjugation but hyperconjugation generally dominates over indirect inductive effect. Aromatic carbocations will be highly stable while antiaromatic carbocations will be highly unstable. See fig.1.
The stability order `3^o > 2^o > 1^o >` methyl can be explained due to positive inductive effect of alkyl groups. Alternatively, it can also be explained on the basis of hyperconjugation effect with `9`, `5`, `3` and zero hyper conjugation in above `3^o , 2^o, 1^o` and methyl carbocations. See fig.2.
The stability order `3^o > 2^o > 1^o >` methyl can be explained due to positive inductive effect of alkyl groups. Alternatively, it can also be explained on the basis of hyperconjugation effect with `9`, `5`, `3` and zero hyper conjugation in above `3^o , 2^o, 1^o` and methyl carbocations. See fig.2.