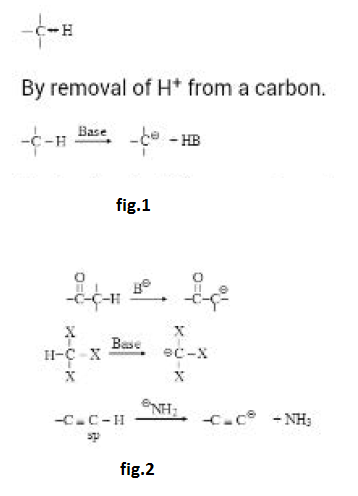
Stability Of Carbanions :
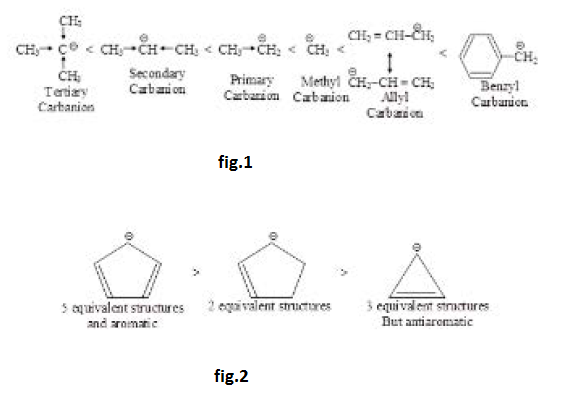
See fig.1.
The stability order `3° < 2° < 1° <` methyl can be explained on the basis of `+I` effect of alkyl groups (electron-releasing) which increases `-ve` charge and decreases stability. The stability of allyl and benzyl carbanions is due to resonance. See fig.2.
The stability order `3° < 2° < 1° <` methyl can be explained on the basis of `+I` effect of alkyl groups (electron-releasing) which increases `-ve` charge and decreases stability. The stability of allyl and benzyl carbanions is due to resonance. See fig.2.