Nitrogen `(N_2)` :
Preparation of `N_2` :
(i) `NH_4NO_2 overset(Delta)-> N_2 + 2H_2O`
(ii) `(NH_4)_2 Cr_2O_7 overset(Delta)-> N_2 + 4H_2O + Cr_2O_3`
(iii) `Ba(N_3)_2 overset(Delta)-> Ba + 3N_2 }` Purest `N_2` obtained
`2NaN_3 -> 2Na + 3N_2` by this method
(iv) `2NH_3 + 3NaOCl -> N_2 + 3NaCl + 3H_2O`
(v) `undersettext(red, overheated)(2NO + 2Cu) -> undersettext(Black)(2CuO + N_2)`
(vi) `Cl_2` passed into liquor `NH_3`
`3Cl_2 + 2NH_3 -> N_2 + 6HCl`
`6NH_3 + 6HCl -> 6NH_4Cl`
`3Cl_2 + 8NH_3 -> N_2 + 6NH_4Cl`
In this method `NH_3` conc. should not be lowered down beyond a particular limit.
`3Cl_2 + NH_3 -> undersettext(Trimendously explosive)(NCl_3 + 3HCl)`
`text(Properties of)` `N_2` :
(i) It is inert due to high bond energy.
(ii) It is absorbed by hot metal like `Ca`, `Mg`, `Al` etc.
`3Ca + N_2 -> Ca_3N_2`
Bright hot `2Al + N_2 -> 2AlN`
`Al_2O_3 + 3C + N_2 -> 2AlN + 3CO`
`(BN)_x` : Inorganic graphite
White slippery solid having `20`-sheet structure.
`(BN)_x overset(3000^oC)-> (BN)_x`
`3`-`D` network structure similar to diamond (Borazon) which is harder than diamond and used for diamond cutting.
`Na_2B_4O_7 + 2NH_4Cl overset(Delta)-> 2NaCl + 2NH_3 + 2B_2O_3 + H_2O`
`B_2O_3 + 2NH_3 -> 2BN + 3H_2O`
(iii) `N_2` can be absorbed by calcium carbide at the temp around `1000^oC`
`undersettext(cynamide ion)(CaC_2 + N_2) overset(1000^oC)-> undersettext(nitrolim)[ul(CaNCN + C)]`
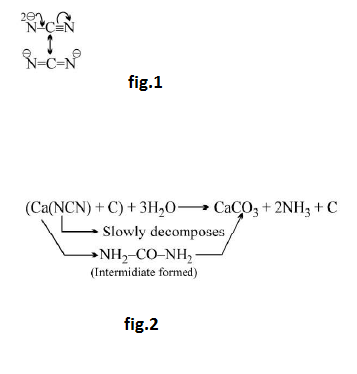
Cynamide ion : See fig.1.
(iv) See fig.2.
`text(Types of Nitride :)`
(i) Salt like or ionic : `Li_3N`, `Na_3N`, `K_3N`, `Ca_3N_2`, `Mg_3N_2`, `Be_3N_2`
(ii) Covalent : `AlN`, `BN`, `Si_3N_4`, `Ge_3N_4`, `Sn_3N_4`
(iii) Interstitial : `MN` `undersettext(HCP or FC C)(`M = Sc`, `Ti`, `Zr`, `Hf`, `La`)
No. of metal atom per unit cell is equal to no. of octahedral voids per unit cell. All the octahedral voids are occupied by nitrogen atoms. Hence the formula is `MN`.
HCP : Hexagonal closed pack
FCC : Face centered cubic
(i) `NH_4NO_2 overset(Delta)-> N_2 + 2H_2O`
(ii) `(NH_4)_2 Cr_2O_7 overset(Delta)-> N_2 + 4H_2O + Cr_2O_3`
(iii) `Ba(N_3)_2 overset(Delta)-> Ba + 3N_2 }` Purest `N_2` obtained
`2NaN_3 -> 2Na + 3N_2` by this method
(iv) `2NH_3 + 3NaOCl -> N_2 + 3NaCl + 3H_2O`
(v) `undersettext(red, overheated)(2NO + 2Cu) -> undersettext(Black)(2CuO + N_2)`
(vi) `Cl_2` passed into liquor `NH_3`
`3Cl_2 + 2NH_3 -> N_2 + 6HCl`
`6NH_3 + 6HCl -> 6NH_4Cl`
`3Cl_2 + 8NH_3 -> N_2 + 6NH_4Cl`
In this method `NH_3` conc. should not be lowered down beyond a particular limit.
`3Cl_2 + NH_3 -> undersettext(Trimendously explosive)(NCl_3 + 3HCl)`
`text(Properties of)` `N_2` :
(i) It is inert due to high bond energy.
(ii) It is absorbed by hot metal like `Ca`, `Mg`, `Al` etc.
`3Ca + N_2 -> Ca_3N_2`
Bright hot `2Al + N_2 -> 2AlN`
`Al_2O_3 + 3C + N_2 -> 2AlN + 3CO`
`(BN)_x` : Inorganic graphite
White slippery solid having `20`-sheet structure.
`(BN)_x overset(3000^oC)-> (BN)_x`
`3`-`D` network structure similar to diamond (Borazon) which is harder than diamond and used for diamond cutting.
`Na_2B_4O_7 + 2NH_4Cl overset(Delta)-> 2NaCl + 2NH_3 + 2B_2O_3 + H_2O`
`B_2O_3 + 2NH_3 -> 2BN + 3H_2O`
(iii) `N_2` can be absorbed by calcium carbide at the temp around `1000^oC`
`undersettext(cynamide ion)(CaC_2 + N_2) overset(1000^oC)-> undersettext(nitrolim)[ul(CaNCN + C)]`
Cynamide ion : See fig.1.
(iv) See fig.2.
`text(Types of Nitride :)`
(i) Salt like or ionic : `Li_3N`, `Na_3N`, `K_3N`, `Ca_3N_2`, `Mg_3N_2`, `Be_3N_2`
(ii) Covalent : `AlN`, `BN`, `Si_3N_4`, `Ge_3N_4`, `Sn_3N_4`
(iii) Interstitial : `MN` `undersettext(HCP or FC C)(`M = Sc`, `Ti`, `Zr`, `Hf`, `La`)
No. of metal atom per unit cell is equal to no. of octahedral voids per unit cell. All the octahedral voids are occupied by nitrogen atoms. Hence the formula is `MN`.
HCP : Hexagonal closed pack
FCC : Face centered cubic