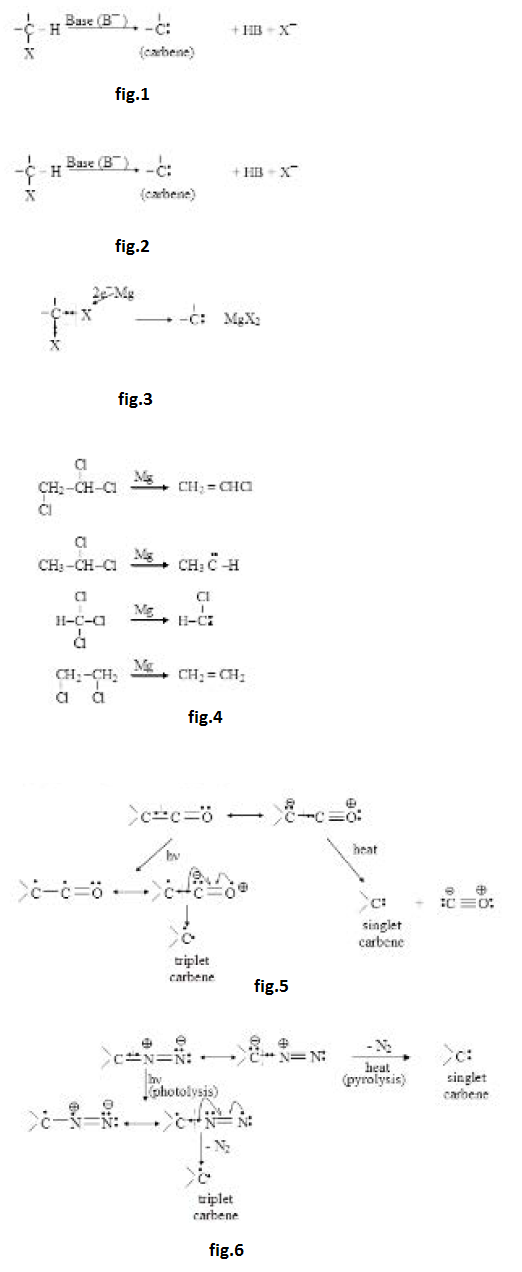
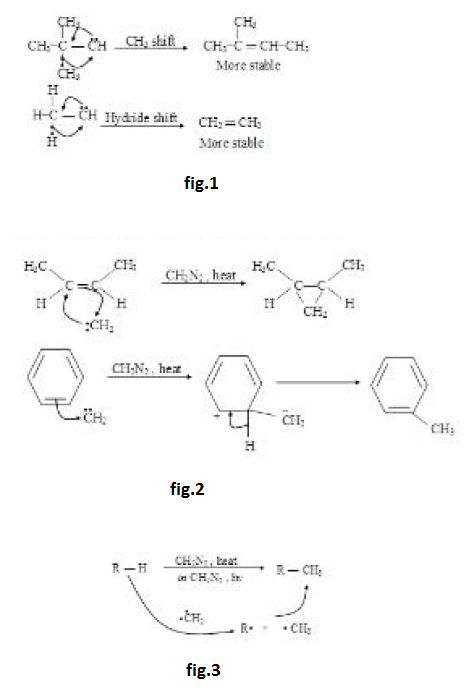
Carbene :

It is a species having a neutral carbon with two bond pairs and two unshared electrons. It is of two types : Singlet and triplet.
(i) Singlet : Total spin `= + 1/2 - 1/2 = 0,` only one value.
`-underset(..)overset(|) C`
(ii) Triplet : It is more stable according to Hund's rule.
`+ 1/2 + 1/2 ,` total spin `= 1`
`-1/2 - 1/2 ,` total spin `= -1` Three different values of total spin.
`-1/2 +1/2 ,` total spin `= 0`
`-underset(.)overset(|)C*`
Both the unpaired electrons have parallel spins in ground state of triplet carbene. See fig.
Carbenes are neutral intermediates having bivalent carbon, in which a carbon atom is covalently bonded to two other groups and has two valency electrons distributed between two non bonding orbitals. When the two electrons have paired spin the carbene is a singlet, if the spins of the electrons are parallel it is a triplet.
(i) Singlet : Total spin `= + 1/2 - 1/2 = 0,` only one value.
`-underset(..)overset(|) C`
(ii) Triplet : It is more stable according to Hund's rule.
`+ 1/2 + 1/2 ,` total spin `= 1`
`-1/2 - 1/2 ,` total spin `= -1` Three different values of total spin.
`-1/2 +1/2 ,` total spin `= 0`
`-underset(.)overset(|)C*`
Both the unpaired electrons have parallel spins in ground state of triplet carbene. See fig.
Carbenes are neutral intermediates having bivalent carbon, in which a carbon atom is covalently bonded to two other groups and has two valency electrons distributed between two non bonding orbitals. When the two electrons have paired spin the carbene is a singlet, if the spins of the electrons are parallel it is a triplet.