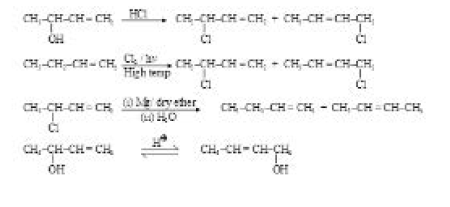
Shifting of a Group from one Atom to the other Atom :
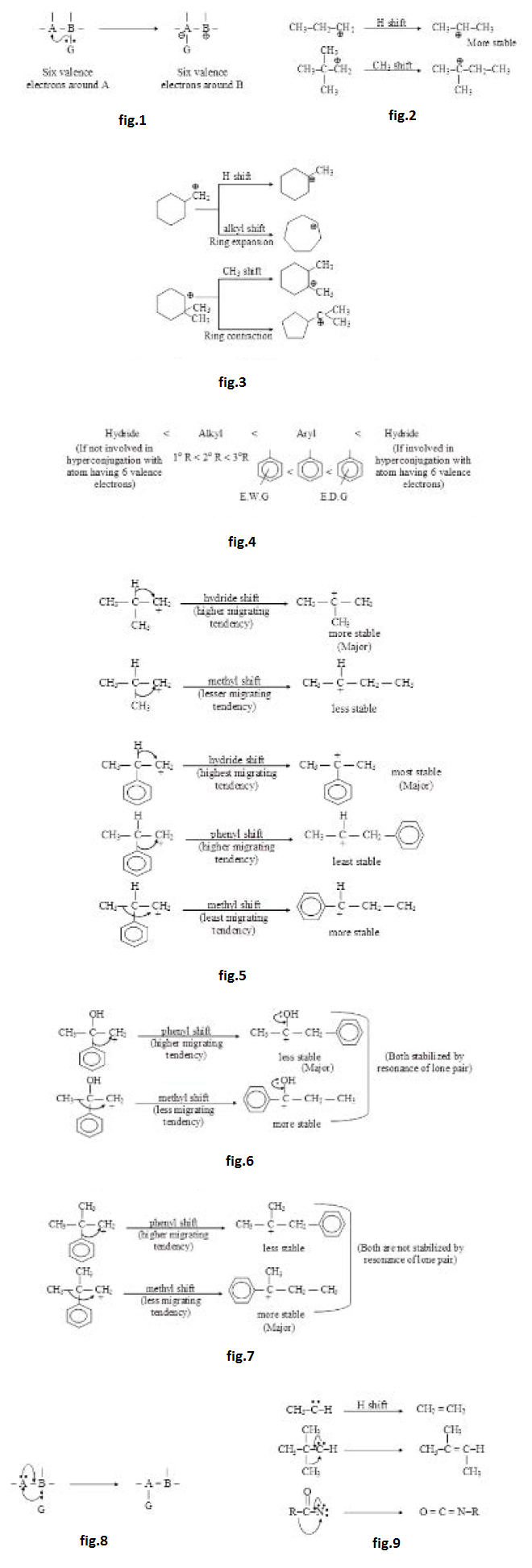
Although shifting of a group can occur from an atom to any other atom yet most of the shifts occur to adjacent atoms only. The main reason for this shifting is presence of six valence electrons and a vacant orbital on an atom. Such an atom has tendency to complete its octet for which the group from the adjacent atom migrates with the bonding electrons. There are two different conditions around an atom with six valence electrons.
(a) `text(When atom with six valence electrons and a vacant orbital :)` See fig.1.
In this case when a group is shifted from atom `B`, the octet of `B` becomes incomplete which is not favourable condition. So we can say that this shift will occur only if it increases stability. See fig.2.
Ring expansion and ring contraction can also result because of adjacent shift. See fig.3.
If migration of two (or more) different groups from adjacent atoms can take place, major migration depends on the migrating tendency of the migrating groups (migration of group with higher migrating tendency is favoured) and the stability of resultant species (more stable resultant species is more favoured). Migrating tendency of a group is generally higher if it is more electron rich i.e. more electron donating. The general order of migrating tendency (or migratory aptitude) is as given in fig.4.
In several cases migrating tendency and the stability of resultant species favours the same migration. In such cases it will be easier to predict the major migration product. See fig.5.
(However, if migrating tendency and stability of resultant species oppose each other, following logics can be applied to predict the major product.)
(i) If both resultant species are very highly stabilized (stabilized by resonance of lone pair or stabilized by formation of additional bond), small amount of stability does not matter a lot. Therefore, major migration occurs according to migrating aptitude. See fig.6.
(ii) If both resultant species are not very highly stabilized (not stabilized by resonance of lone pair or not stabilized by formation of additional bond), small amount of stability also matters a lot. Therefore, major migration occurs according to stability of resultant species. See fig.7.
(iii) If the difference of stability is large, stability will dominate over the migrating tendency. Therefore, groups containing lone pairs migrate rarely.
(b) `text(When atom with six valence electrons and a vacant orbital also has lone pair)`
See fig.8.
In this case when a group from atom `B` is shifted, octet of `B` becomes incomplete which can be completed (stabilized) by lone pairs present on `A` atom. Hence under these conditions stability increases always and these rearrangements are, in general, bound to take place in the given species. Moreover, in such cases formation of additional bond or stabilization by resonance of lone pair will be generally observed, therefore migrating tendency generally dominates. See fig.9.
(a) `text(When atom with six valence electrons and a vacant orbital :)` See fig.1.
In this case when a group is shifted from atom `B`, the octet of `B` becomes incomplete which is not favourable condition. So we can say that this shift will occur only if it increases stability. See fig.2.
Ring expansion and ring contraction can also result because of adjacent shift. See fig.3.
If migration of two (or more) different groups from adjacent atoms can take place, major migration depends on the migrating tendency of the migrating groups (migration of group with higher migrating tendency is favoured) and the stability of resultant species (more stable resultant species is more favoured). Migrating tendency of a group is generally higher if it is more electron rich i.e. more electron donating. The general order of migrating tendency (or migratory aptitude) is as given in fig.4.
In several cases migrating tendency and the stability of resultant species favours the same migration. In such cases it will be easier to predict the major migration product. See fig.5.
(However, if migrating tendency and stability of resultant species oppose each other, following logics can be applied to predict the major product.)
(i) If both resultant species are very highly stabilized (stabilized by resonance of lone pair or stabilized by formation of additional bond), small amount of stability does not matter a lot. Therefore, major migration occurs according to migrating aptitude. See fig.6.
(ii) If both resultant species are not very highly stabilized (not stabilized by resonance of lone pair or not stabilized by formation of additional bond), small amount of stability also matters a lot. Therefore, major migration occurs according to stability of resultant species. See fig.7.
(iii) If the difference of stability is large, stability will dominate over the migrating tendency. Therefore, groups containing lone pairs migrate rarely.
(b) `text(When atom with six valence electrons and a vacant orbital also has lone pair)`
See fig.8.
In this case when a group from atom `B` is shifted, octet of `B` becomes incomplete which can be completed (stabilized) by lone pairs present on `A` atom. Hence under these conditions stability increases always and these rearrangements are, in general, bound to take place in the given species. Moreover, in such cases formation of additional bond or stabilization by resonance of lone pair will be generally observed, therefore migrating tendency generally dominates. See fig.9.