Mechanism of Halogenation of Alkanes :
`text(Halogenation :)` See fig.1.
`undersettext(excess)(CH_4) + X_2 overset(U.V.)-> CH_3-X +HX`
`CH_4 +X_2 overset(U.V.)-> CH_3-X -> CH_2X_2-> CHX_3 -> CX_4`
`undersettext(excess)(CH_3-CH_3) +Cl_2 oversettext(sunlight)oversettext(diffused)-> CH_3CH -Cl + HCl`
`CH_3-CH_3 + undersettext(excess)(Cl_2) undersettext(sunlight)oversettext(diffused)-> CH_3CH_2Cl +` mixture of product obtained by polychlorination
See fig.2.
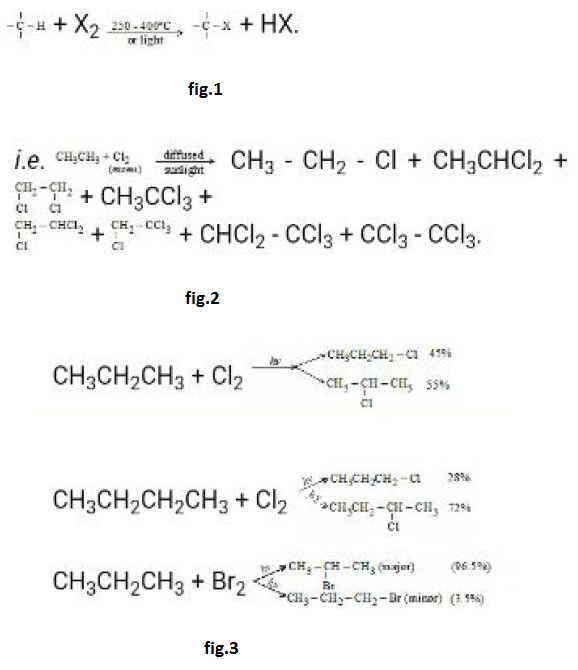
Mono halogenation of propane and Butane : See fig.3.
Hence these reactions show that the reaction depends upon the abstracting power of the halogen atoms as well as the number of `H`-atoms present in the organic molecule.
The abstracting power of the halogen atoms refers to the selectivity factor which includes the `HX` bond energy as well as bond dissociation energy of the `C-H` bond.
`undersettext(excess)(CH_4) + X_2 overset(U.V.)-> CH_3-X +HX`
`CH_4 +X_2 overset(U.V.)-> CH_3-X -> CH_2X_2-> CHX_3 -> CX_4`
`undersettext(excess)(CH_3-CH_3) +Cl_2 oversettext(sunlight)oversettext(diffused)-> CH_3CH -Cl + HCl`
`CH_3-CH_3 + undersettext(excess)(Cl_2) undersettext(sunlight)oversettext(diffused)-> CH_3CH_2Cl +` mixture of product obtained by polychlorination
See fig.2.
Mono halogenation of propane and Butane : See fig.3.
Hence these reactions show that the reaction depends upon the abstracting power of the halogen atoms as well as the number of `H`-atoms present in the organic molecule.
The abstracting power of the halogen atoms refers to the selectivity factor which includes the `HX` bond energy as well as bond dissociation energy of the `C-H` bond.
