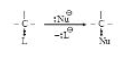
See fig.1.
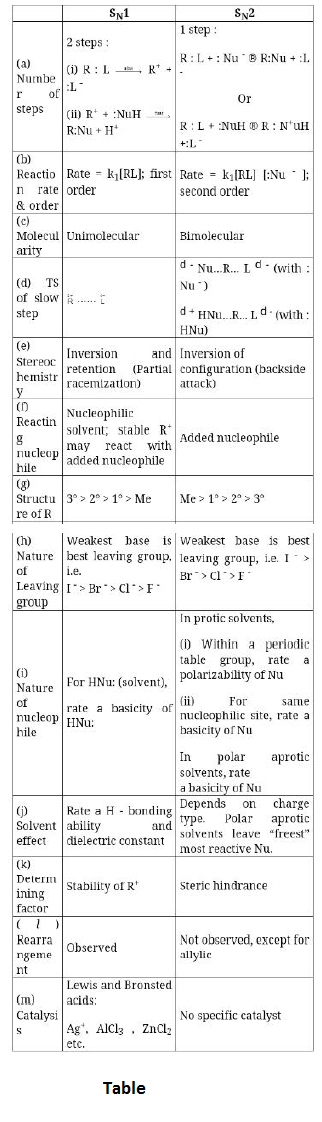
In unimolecular Nucleophilic substitution reaction, leaving group (a nucleophile) is first expelled in slow (rate determining) step followed by attack of another nucleophile. Some important characteristics of `SN^1` mechanism are :
(i) Better the leaving group, faster will be rare of `SN^1` reaction because leaving group is expelled in rate determining step.
`R-I > R-Br > R-Cl` (`SN^1` reactivity)
(ii) As the product of rate determining step is carbocation, we can interpret that more the stability of carbocation, more will be rate of `SN^1` reaction.
`3° R-X > 2° R-X > 1° R-X`
`3° R-OH > 2° R-OH > 1° R-OH`
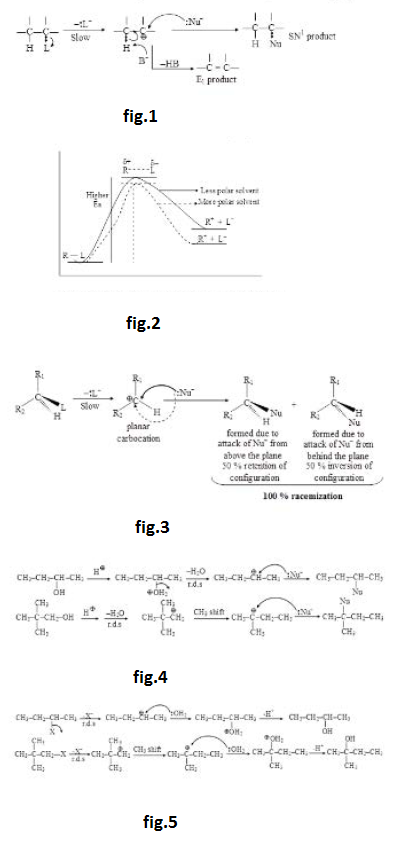
(iii) When solvent polarity is increased, increase in solvation of neutral reactant is almost unaffected, solvation of partially charged transition state increases slightly but the solvation of charged intermediate increases considerably (as shown in energy profile of rate determining step). Therefore, increase in solvent polarity decreases the activation energy and increases the rate of `SN^1` reaction. Hence, `SN^1` reaction is highly favoured in polar solvent. See fig.2.
(iv) As a carbocation (containing six valence electrons and a vacant orbital) is formed, rearrangements are possible before the attack of nucleophile.
(v) `Nu^(-)` can be a weaker nucleophile than `L^(-)`. A strong `Nu^-` is not required for `SN^(1)` reaction. Moreover, increase in nucleophilic strength of `Nu^(-)` or its concentration will not increase the rate of `SN^(1)` reaction.
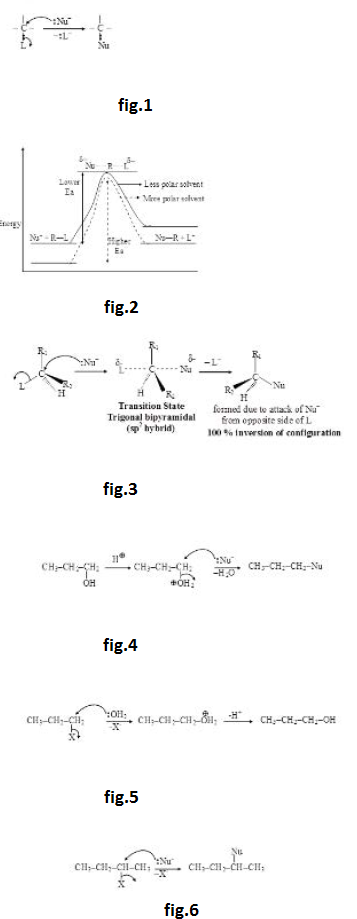
(vi) As carbocation is planar, attack of `Nu^-` from both the sides of plane is equally favoured. Therefore, `SN^1` reaction proceeds with 50% inversion of configuration (attack of nucleophile from opposite side of leaving group) and 50% retention of configuration (attack of nucleophile from the side of leaving group) i.e. `SN^1` reaction proceeds with 100% racemization (racemization is the process of formation of racemic mixture which is the equilibrium mixture of two mirror images of each other). See fig.3.
However, it must be remembered that even if solvent is highly polar (which is generally used for doing `SN^1` reactions), the attractive force between the carbocation and `L^-` cannot be completely destroyed. They will have some attractive force and will have some extent of ion pairing (although very small). Therefore, attack of nucleophile from opposite side of leaving group will be more favoured (though very slightly). In other words, inversion of configuration will be slightly more than retention of configuration i.e. `S_N 1` reaction leads to almost 100% racemization with little inversion.
(vii) Unimolecular elimination `(E_1)` will be the competing reaction.
`text(Examples of)` `SN^1 :`
`(i)` `text(Acid catalyzed nucleophilic substitution on most alcohols except smaller primary alcohols :)` See fig.4.
`(ii)` `text(Solvolysis)` `text((hydrolysis & alcoholysis))` `text(of most Alkyl halides except smaller)` `1^o` `text(Alkyl halides.)` See fig.5.
See fig.1.
In unimolecular Nucleophilic substitution reaction, leaving group (a nucleophile) is first expelled in slow (rate determining) step followed by attack of another nucleophile. Some important characteristics of `SN^1` mechanism are :
(i) Better the leaving group, faster will be rare of `SN^1` reaction because leaving group is expelled in rate determining step.
`R-I > R-Br > R-Cl` (`SN^1` reactivity)
(ii) As the product of rate determining step is carbocation, we can interpret that more the stability of carbocation, more will be rate of `SN^1` reaction.
`3° R-X > 2° R-X > 1° R-X`
`3° R-OH > 2° R-OH > 1° R-OH`
(iii) When solvent polarity is increased, increase in solvation of neutral reactant is almost unaffected, solvation of partially charged transition state increases slightly but the solvation of charged intermediate increases considerably (as shown in energy profile of rate determining step). Therefore, increase in solvent polarity decreases the activation energy and increases the rate of `SN^1` reaction. Hence, `SN^1` reaction is highly favoured in polar solvent. See fig.2.
(iv) As a carbocation (containing six valence electrons and a vacant orbital) is formed, rearrangements are possible before the attack of nucleophile.
(v) `Nu^(-)` can be a weaker nucleophile than `L^(-)`. A strong `Nu^-` is not required for `SN^(1)` reaction. Moreover, increase in nucleophilic strength of `Nu^(-)` or its concentration will not increase the rate of `SN^(1)` reaction.
(vi) As carbocation is planar, attack of `Nu^-` from both the sides of plane is equally favoured. Therefore, `SN^1` reaction proceeds with 50% inversion of configuration (attack of nucleophile from opposite side of leaving group) and 50% retention of configuration (attack of nucleophile from the side of leaving group) i.e. `SN^1` reaction proceeds with 100% racemization (racemization is the process of formation of racemic mixture which is the equilibrium mixture of two mirror images of each other). See fig.3.
However, it must be remembered that even if solvent is highly polar (which is generally used for doing `SN^1` reactions), the attractive force between the carbocation and `L^-` cannot be completely destroyed. They will have some attractive force and will have some extent of ion pairing (although very small). Therefore, attack of nucleophile from opposite side of leaving group will be more favoured (though very slightly). In other words, inversion of configuration will be slightly more than retention of configuration i.e. `S_N 1` reaction leads to almost 100% racemization with little inversion.
(vii) Unimolecular elimination `(E_1)` will be the competing reaction.
`text(Examples of)` `SN^1 :`
`(i)` `text(Acid catalyzed nucleophilic substitution on most alcohols except smaller primary alcohols :)` See fig.4.
`(ii)` `text(Solvolysis)` `text((hydrolysis & alcoholysis))` `text(of most Alkyl halides except smaller)` `1^o` `text(Alkyl halides.)` See fig.5.