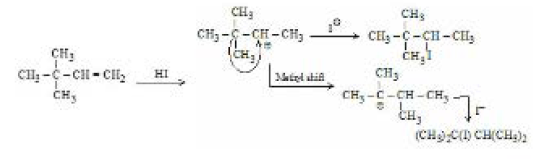
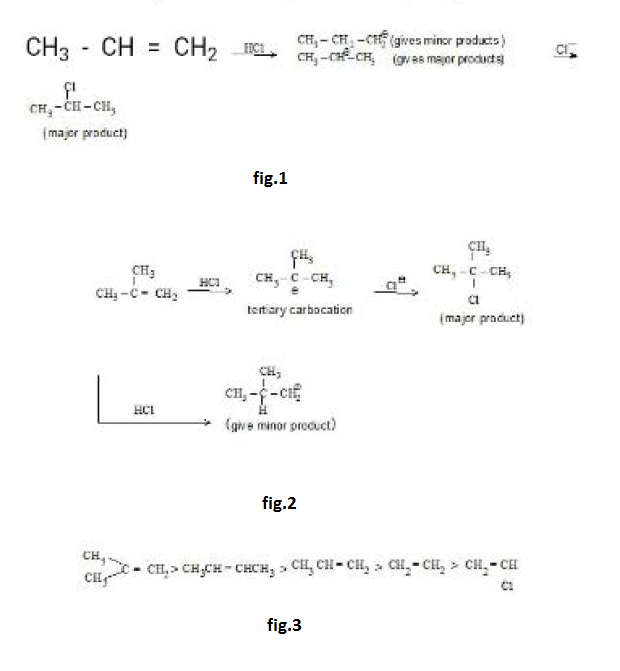
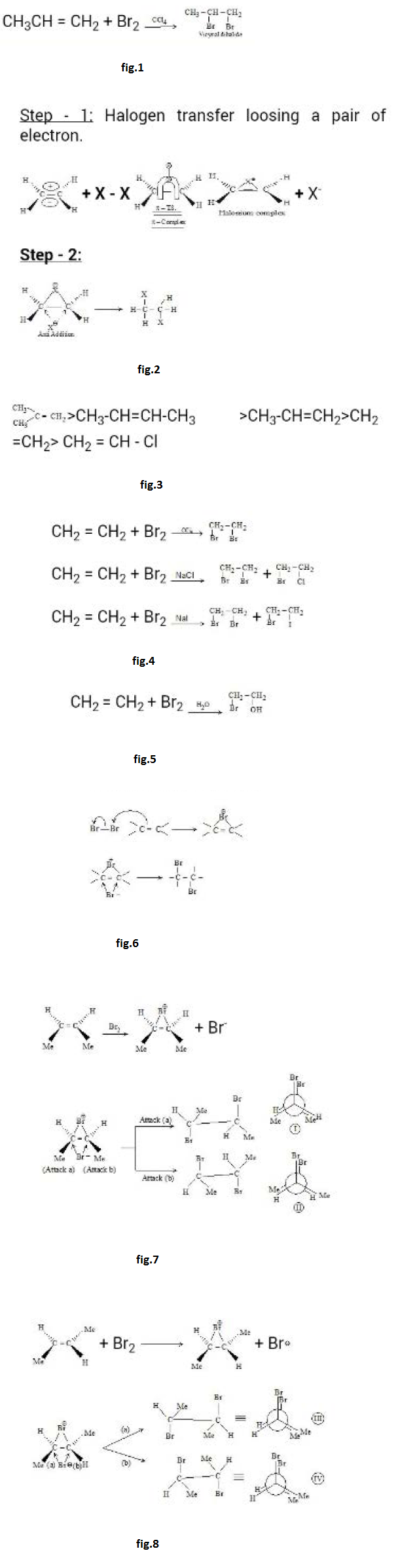
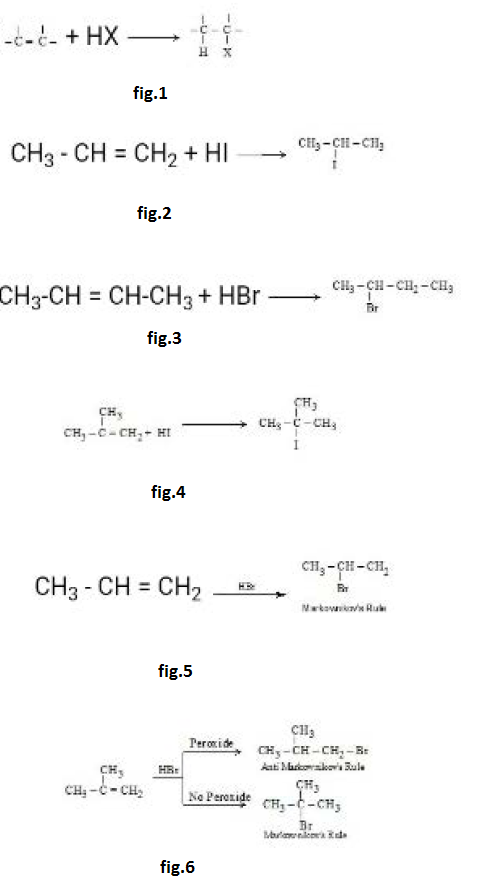
Reactions at the Carbon - Carbon Double Bond :
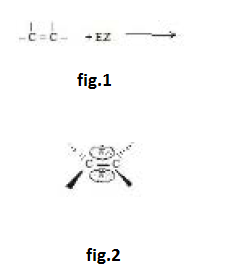
`text(Addition Reaction :)` The double bond consists of a strong `sigma` bond and a weak `pi` bond; we expect, therefore, that reaction would involve breaking of this weaker bond. This expectation is correct; the typical reactions of the double bond are of the sort where the `pi` bond is broken and two strong `sigma` bonds are formed in its place. See fig.1.
A reaction in which two molecules combine to yield a single molecule or product is called as an addition reaction. The reagent is simply added to the substrate, in contrast to a substitution reaction where part of the reagent is substituted for a part of the substrate. Addition reactions are necessarily limited to compounds that contain atoms sharing more than one pair of electrons that is, to compounds that contain multiple bonded atoms. Formally, addition is the opposite of elimination; just as elimination generates a multiple bond, addition destroys it. In the structure of the bond there is a cloud of `pi` electrons above and below the plane of the atoms. These `pi` electrons are less involved than the `sigma` electrons in holding together the carbon nuclei. See fig.2.
As a result, they are themselves held less tightly. These loosely held electrons are particularly available to a reagent that is seeking electrons. It is not surprising, then, that in many of its reactions the carbon-carbon double bond serves as a source of electrons : that is, it acts as a base. The compounds with which it reacts are those that are deficient in electrons. These acidic reagents that are seeking a pair of electrons are called electrophilic reagents. The typical reaction of an alkene is electrophilic addition, or, in other words, addition of acidic reagents. Reagents of another kind i.e., free radicals also seek electrons or, rather, seek an electron. And so we find that alkenes also undergo free-radical addition.
A reaction in which two molecules combine to yield a single molecule or product is called as an addition reaction. The reagent is simply added to the substrate, in contrast to a substitution reaction where part of the reagent is substituted for a part of the substrate. Addition reactions are necessarily limited to compounds that contain atoms sharing more than one pair of electrons that is, to compounds that contain multiple bonded atoms. Formally, addition is the opposite of elimination; just as elimination generates a multiple bond, addition destroys it. In the structure of the bond there is a cloud of `pi` electrons above and below the plane of the atoms. These `pi` electrons are less involved than the `sigma` electrons in holding together the carbon nuclei. See fig.2.
As a result, they are themselves held less tightly. These loosely held electrons are particularly available to a reagent that is seeking electrons. It is not surprising, then, that in many of its reactions the carbon-carbon double bond serves as a source of electrons : that is, it acts as a base. The compounds with which it reacts are those that are deficient in electrons. These acidic reagents that are seeking a pair of electrons are called electrophilic reagents. The typical reaction of an alkene is electrophilic addition, or, in other words, addition of acidic reagents. Reagents of another kind i.e., free radicals also seek electrons or, rather, seek an electron. And so we find that alkenes also undergo free-radical addition.