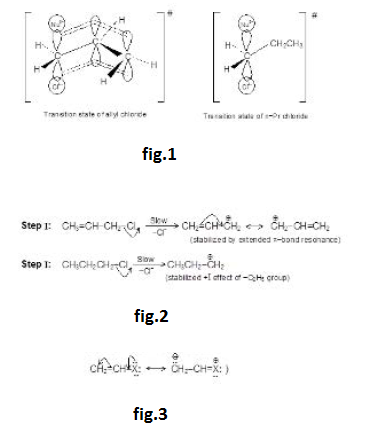
Substitution vs. Elimination Reaction :
We know that an alkyl ha lide can undergo four types of reactions : `S_N1, S_N2, E_1` and `E_2`.
A given alkyl halide under the given conditions will follow which pathway, can be predicted in following manner.
The first thing you must look at is the alkyl halide, is it `1^o, 2^o` or `3^o`. lf the reactant were a primary alkyl halide, it would undergo `(E_2)//(S_N2)` reactions (as their carbocations are not stable).
If the reactant is a secondary or a tertiary alkyl halide, then it can undergo `E_1//(S_N1 )` or `E_2//(S_N2)` reactions depending upon reaction conditions. `E_2//S_N2` reactions are favoured by a high concentration of a good nucleophile/Strong base, whereas a poor nucleophile{Weak base favours `E_1//(S_N1)` reactions.
Once you have decided whether the conditions will favour `E_2//(S_N2)` reactions or `E_1//(S_N1)` reactions, then you should decide how much of the product will be substitution and how much will be the elimination product. The relative amount of substitution and elimination product can be decided again on the basis of structure of alkyl halide (i.e. `1°`, `2°` or `3°`) and on the nature of the nucleophile/base. Relative reactivities of alkyl halides in various reactions are :
In an `S_N2` reaction : `1^o > 2^o > 3^o`
In an `E_2` reaction : `3^o > 2^o > 1^o`
ln an `S_N1` reaction : `3^o > 2^o > 1^o`
ln an `E_1` reaction : `3^o > 2^o > 1^o`
For instance, propyl bromide when treated with methoxide ion in methanol can undergo either substitution reaction to give methyl propyl ether or elimination reaction to give propene.
The major product of the reaction would be substitution product.
`CH_3CH_2CH_2 - Br + CH_3O overset(CH_3OH)-> CH_3CH_2CH_2OCH_3 + underset(90%)(CH_3CH=CH_2) + underset(10%)(CH_3OH) + Br^(-)`
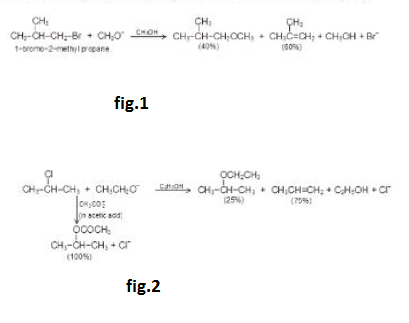
But when the primary alkyl halide or the nucleophile base is sterically hindered, the nucleophile will have difficulty getting to the back of `alpha`-carbon and thus, elimination product will predominate. For example, See fig.1.
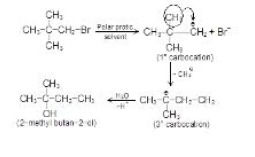
A secondary alkyl halide can form both substitution and elimination products, whose relative amotmt depend on the base strength of the nucleophile. The stronger and bulkier the base, greater will be the percent of the elimination product. See fig.2.
Increasing the temperature at which the reaction is carried out increases the rates of both the substitution and elimination reactions but increase in the rate of elimination reaction is more than that of substitution reaction. Thus, if the substitution product is desired, the reaction should be carried out at low temperature and high temperature promotes elimination product.
A tertiary alkyl halide is least reactive towards `S_N2` reaction but most reactive towards `E_2` reaction. Thus, only elimination product is formed.
When the nucleophile is poor or base is weak, `E_1//(S_N1)` reactions will be preferred. Both `E_1//(S_N1)` reactions will take place through the formation of carbocation, formed by the heterolytic dissociation of alkyl halide. Alkyl halides have the same order of reactivity in `E_1` and `S_N1` reaction because they have the same rate - determining step. Thus, all alkyl halides that react by `E_1//(S_N1)` reactions would give both elimination and substitution products. Substitution is favoured over elimination at lower temperatures and with the increase of temperature, the percentage of elimination product increases. Primary alkyl halides do not undergo `E_1//S_N1` reactions because primary carbocations are not too stable.
A given alkyl halide under the given conditions will follow which pathway, can be predicted in following manner.
The first thing you must look at is the alkyl halide, is it `1^o, 2^o` or `3^o`. lf the reactant were a primary alkyl halide, it would undergo `(E_2)//(S_N2)` reactions (as their carbocations are not stable).
If the reactant is a secondary or a tertiary alkyl halide, then it can undergo `E_1//(S_N1 )` or `E_2//(S_N2)` reactions depending upon reaction conditions. `E_2//S_N2` reactions are favoured by a high concentration of a good nucleophile/Strong base, whereas a poor nucleophile{Weak base favours `E_1//(S_N1)` reactions.
Once you have decided whether the conditions will favour `E_2//(S_N2)` reactions or `E_1//(S_N1)` reactions, then you should decide how much of the product will be substitution and how much will be the elimination product. The relative amount of substitution and elimination product can be decided again on the basis of structure of alkyl halide (i.e. `1°`, `2°` or `3°`) and on the nature of the nucleophile/base. Relative reactivities of alkyl halides in various reactions are :
In an `S_N2` reaction : `1^o > 2^o > 3^o`
In an `E_2` reaction : `3^o > 2^o > 1^o`
ln an `S_N1` reaction : `3^o > 2^o > 1^o`
ln an `E_1` reaction : `3^o > 2^o > 1^o`
For instance, propyl bromide when treated with methoxide ion in methanol can undergo either substitution reaction to give methyl propyl ether or elimination reaction to give propene.
The major product of the reaction would be substitution product.
`CH_3CH_2CH_2 - Br + CH_3O overset(CH_3OH)-> CH_3CH_2CH_2OCH_3 + underset(90%)(CH_3CH=CH_2) + underset(10%)(CH_3OH) + Br^(-)`
But when the primary alkyl halide or the nucleophile base is sterically hindered, the nucleophile will have difficulty getting to the back of `alpha`-carbon and thus, elimination product will predominate. For example, See fig.1.
A secondary alkyl halide can form both substitution and elimination products, whose relative amotmt depend on the base strength of the nucleophile. The stronger and bulkier the base, greater will be the percent of the elimination product. See fig.2.
Increasing the temperature at which the reaction is carried out increases the rates of both the substitution and elimination reactions but increase in the rate of elimination reaction is more than that of substitution reaction. Thus, if the substitution product is desired, the reaction should be carried out at low temperature and high temperature promotes elimination product.
A tertiary alkyl halide is least reactive towards `S_N2` reaction but most reactive towards `E_2` reaction. Thus, only elimination product is formed.
When the nucleophile is poor or base is weak, `E_1//(S_N1)` reactions will be preferred. Both `E_1//(S_N1)` reactions will take place through the formation of carbocation, formed by the heterolytic dissociation of alkyl halide. Alkyl halides have the same order of reactivity in `E_1` and `S_N1` reaction because they have the same rate - determining step. Thus, all alkyl halides that react by `E_1//(S_N1)` reactions would give both elimination and substitution products. Substitution is favoured over elimination at lower temperatures and with the increase of temperature, the percentage of elimination product increases. Primary alkyl halides do not undergo `E_1//S_N1` reactions because primary carbocations are not too stable.