`text(Addition of)` `H_2SO_4` : Alkenes react with cold concentrated sulphuric acid to form compounds of general formula `ROSO_3 H`. known as alkyl hydrogen sulphates. The reactions are again highly regeioselective and follow the Markownikov's Rule.
`CH_2=CH_2 overset(98 % H_2SO_4)-> undersettext(Ethyl hydrogen Sulfate)(CH_3CH_2OSO_3H)`
See fig.1.
The alkyl hydrogen sulphates are deliquiscent solids and are difficult to isolate.
When such an alkyl hydrogen sulphate is diluted with water and heated, we get an alcohol bearing the same alkyl group. The ester of the sulphuric acid is cleaved to get an alcohol and sulfuric acid.
`CH_2 = CH_2 overset(98 % H_2SO_4)->CH_3CH_2 oSO_3H underset(Delta)overset(H_2O)-> CH_3CH_2 - OH +H_2SO_4`
See fig.2.
This sequence of the reaction provides a good route for preparing alcohols.
`text(Addition of) H_2O :` Water may be added to alkenes in the presence of acids to yield alcohols. This reaction also follows Markownikov's rule.
`CH_2=CH_2 +H_2O CH_3 -CH_2-OH +H_2O`
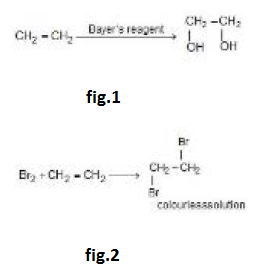
`text(Addition of Hypohalous Acids :)` The addition of `Cl_2` or `Br_2` in the presence of water can yield compounds containing halogen and `OH` on adjacent `C`-atoms such compounds are called halohydrins.
See fig.3.
These compounds are not formed due to the formation of `HOX` but by reactions of alkene with halogen and water.
See fig.4.
These reactions follow anti stereospecific mechanism.
Though these reactions proceed to form products which are in accordance with Markownikov's rule, the stereochemistry suggests that the reaction proceed via halomium ion formation and not via formation of classical carbocation
`text(Addition of Alkene Dimerization :)`
When lsobutene is reacted with catalytic amount of `H_2SO_4` or `H_3PO_4`
(a protic acid) then we get a mixture of two alkenes with molecular formula `C_8H_(16)`
See fig.5.
`text(Mechanism)` : See fig.6.
`text(Addition of Alkanes (Alkylation) :)` When alkenes are reacted with alkanes in the presence of `H_2SO_4` or `HF` at `0°C` then we get a higher homologue of alkane. See fig.7.
`text(Mechanism :)` See fig.8.
`text(Oxymercuration - Demercuration :)`
Alkenes react with mercuric acetate in the presence of water to give hydroxy mercurial compounds which on reduction yields alcohols.
See fig.9.
Oxy mercuration-Demercuration is highly regeioselective and gives alcohols corresponding to Markownikov's Rule
`CH_3CH_2CH_2CH_2CH =CH_2`
Oxymercuration involves electrophilic addition to carbon-carbon double bond with the mercuric ion acting as electrophile.
See fig.10.
`text(Hydroboration-Oxidation :)` When alkenes are reacted with dibroranes `(B_2H_6)`, alkenes undergo hydroboration to yield alkyl boranes `(R_3B)` which on oxidation give alcohols.
`CH_2 = CH_2 + B_2H_5 -> (CH_3 CH_2 )_3B`
`(CH_3CH_2)_2B + 3H_2O overset(OH^(⊖))-> CH_3CH_2 - OH + B(OH)_3`
All Hydroboration-oxidation, reactions are highly regeioselective, however here the product formed is according to Anti Markownikov's Rule.
`CH_3 - CH = CH_2 + B_2H_6 -> (CH_3CH_2CH_2)_3B underset(OH^(⊖))overset(H_2O_2)->CH_3CH_2CH_2 - OH + H_3BO_3`
`CH_3 CH_2CH = CH_2 + B_2H_6 -> (CH_3CH_2CH_2CH_2 )_3B underset(OH^(⊖))overset(H_2O_2)->CH_3CH_2CH_2CH_2- OH + H_3BO_3`
Thus rearrangement does not occur in Hydroboration.
Oxidation Reactions in Alkene :
(i) `text(Hydroxylation)` (Using Baeyer's Reagent): When an alkene react with dilute alkaline `KMnO_4` solution in cold condition then the alkene gets converted to vicinal diol.
See fig.11.
`text(Mechanism)` : See fig.12.
This reaction on alkene generates vicinal diol and is a SYN-ADDITION reaction.
This is supported by the mechanism that the oxygen atoms of `OH` group in the diol formed are from the permanganate ions which add to the alkene molecule from the same side.
(ii) With `OsO_4` (Osmium tetraoxide) : See fig.13.
This is again a SYN-ADDITION reaction.
(iii) `text(With Hot)` `KMnO_4 :` Alkenes on reaction with hot alkaline `KMnO_4` give a mixture of carboxylic acid and ketones or only ketones or carboxylic acids.
`CH_2=CH_2 underset(KMnO_4)overset([O])-> 2 HCOOH underset(Delta)overset([O])-> 2CO_2 +2H_2O`
`CH_3 -CH =CH_2CH_3COOH +CO_2 +H_2O`
See fig.14.
`text(Addition of Oxygen :)` Alkenes on addition with oxygen in the presence of silver as catalyst at `570` `K` forms epoxide.
See fig.15.
`text(Substitution Reactions :)` Most alkenes contain not only the carbon-carbon double bond but also alkyl groups which have essentially alkane character. Hence besides the addition reactions which are characterstics of carbon-carbon double bonds, Alkenes may undergo the free radical substitution also which is a characteristic of alkanes.
`(a)` `text(Halogenation, Allylic Substitution)`
`H - underset(|)overset(|)C - overset(|)C - overset(|)C - X_2 underset(Delta)-> X - underset(|)overset(|)C - overset(|)C - overset(|)C - `
`CH_3 -CH = CH_2 underset(700^oC)overset(Cl_2)-> Cl - CH_2 -CH = CH_2`
See fig.16.
When we consider the molecule of propene
See fig.17.
The Site-I is the alkene site where due to the presence of reactive `pi`-bonds the reaction can take place at low temperature, in the absence of light and generally in liquid state. While site-II is the alkane site which requires high temperatures or U.V light. If we try to make the reaction possible at site-II, we must take the necessary condition of high temperature or U.V. radiations.
See fig.18.
`text(Mechanism)` :
lnilation `X_2 underset(U . V )oversettext(high temg.)-> 2X^(.)`
`text(Propogations :)`
`X^(.) +CH_3 -CH =CH_2 -> HX + overset(.)(CH_2) - CH =CH_2`
See fig.19.
Also `CH_3-CH -CH_2+SO_2 -> underset(|_(Cl))(CH_2) -CH _CH_2 +HCl +SO_2`
`CH_3 -CH -CH_2Me_5 COCl ->underset(|_(Cl))(CH_2) - CH -CH_2 +HCl`
`text(Addition of Carbenes :)` Alkenes react with diazomethane to form cyclic compounds.
See fig.20.
`text(Mechanism)` : See fig.21.
`text(Addition of Free Radicals :)` Analogous to free radical addition of `HBr` to alkenes, even `C Cl_4` or `CBr Cl_3`, or `CBr_2 Cl_2` can be added on to alkenes by using certain conditions favourable for free radical addition reaction
See fig.22.
`text(Mechanism)` :
Peroxide `->` Radical
Radical `+ C Cl_4 ->` Radical `Cl + overset(.)(CCl_3)`
`overset(.)(C Cl_3) + RCH =CH_2 -> R- overset(.)CH -CH_2 -C Cl_3`
See fig.23.
`text(Isomerization :)`
`CH_3CH_2CH_2CH =CH_3-CH_2 -CH = CH -CH_3CH_2 -CH = -CH_2`
`text(Diets Alder reaction :)` See fig.24.
Presence of electron donating groups on diene and electron withdrawing groups on dienophile favours the reaction.
`text(Addition of)` `H_2SO_4` : Alkenes react with cold concentrated sulphuric acid to form compounds of general formula `ROSO_3 H`. known as alkyl hydrogen sulphates. The reactions are again highly regeioselective and follow the Markownikov's Rule.
`CH_2=CH_2 overset(98 % H_2SO_4)-> undersettext(Ethyl hydrogen Sulfate)(CH_3CH_2OSO_3H)`
See fig.1.
The alkyl hydrogen sulphates are deliquiscent solids and are difficult to isolate.
When such an alkyl hydrogen sulphate is diluted with water and heated, we get an alcohol bearing the same alkyl group. The ester of the sulphuric acid is cleaved to get an alcohol and sulfuric acid.
`CH_2 = CH_2 overset(98 % H_2SO_4)->CH_3CH_2 oSO_3H underset(Delta)overset(H_2O)-> CH_3CH_2 - OH +H_2SO_4`
See fig.2.
This sequence of the reaction provides a good route for preparing alcohols.
`text(Addition of) H_2O :` Water may be added to alkenes in the presence of acids to yield alcohols. This reaction also follows Markownikov's rule.
`CH_2=CH_2 +H_2O CH_3 -CH_2-OH +H_2O`
`text(Addition of Hypohalous Acids :)` The addition of `Cl_2` or `Br_2` in the presence of water can yield compounds containing halogen and `OH` on adjacent `C`-atoms such compounds are called halohydrins.
See fig.3.
These compounds are not formed due to the formation of `HOX` but by reactions of alkene with halogen and water.
See fig.4.
These reactions follow anti stereospecific mechanism.
Though these reactions proceed to form products which are in accordance with Markownikov's rule, the stereochemistry suggests that the reaction proceed via halomium ion formation and not via formation of classical carbocation
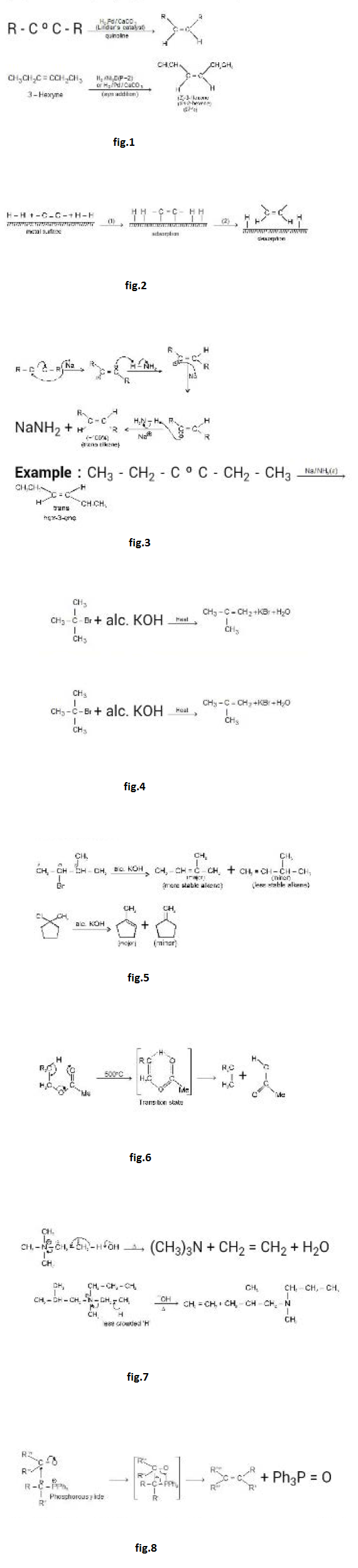
`text(Addition of Alkene Dimerization :)`
When lsobutene is reacted with catalytic amount of `H_2SO_4` or `H_3PO_4`
(a protic acid) then we get a mixture of two alkenes with molecular formula `C_8H_(16)`
See fig.5.
`text(Mechanism)` : See fig.6.
`text(Addition of Alkanes (Alkylation) :)` When alkenes are reacted with alkanes in the presence of `H_2SO_4` or `HF` at `0°C` then we get a higher homologue of alkane. See fig.7.
`text(Mechanism :)` See fig.8.
`text(Oxymercuration - Demercuration :)`
Alkenes react with mercuric acetate in the presence of water to give hydroxy mercurial compounds which on reduction yields alcohols.
See fig.9.
Oxy mercuration-Demercuration is highly regeioselective and gives alcohols corresponding to Markownikov's Rule
`CH_3CH_2CH_2CH_2CH =CH_2`
Oxymercuration involves electrophilic addition to carbon-carbon double bond with the mercuric ion acting as electrophile.
See fig.10.
`text(Hydroboration-Oxidation :)` When alkenes are reacted with dibroranes `(B_2H_6)`, alkenes undergo hydroboration to yield alkyl boranes `(R_3B)` which on oxidation give alcohols.
`CH_2 = CH_2 + B_2H_5 -> (CH_3 CH_2 )_3B`
`(CH_3CH_2)_2B + 3H_2O overset(OH^(⊖))-> CH_3CH_2 - OH + B(OH)_3`
All Hydroboration-oxidation, reactions are highly regeioselective, however here the product formed is according to Anti Markownikov's Rule.
`CH_3 - CH = CH_2 + B_2H_6 -> (CH_3CH_2CH_2)_3B underset(OH^(⊖))overset(H_2O_2)->CH_3CH_2CH_2 - OH + H_3BO_3`
`CH_3 CH_2CH = CH_2 + B_2H_6 -> (CH_3CH_2CH_2CH_2 )_3B underset(OH^(⊖))overset(H_2O_2)->CH_3CH_2CH_2CH_2- OH + H_3BO_3`
Thus rearrangement does not occur in Hydroboration.
Oxidation Reactions in Alkene :
(i) `text(Hydroxylation)` (Using Baeyer's Reagent): When an alkene react with dilute alkaline `KMnO_4` solution in cold condition then the alkene gets converted to vicinal diol.
See fig.11.
`text(Mechanism)` : See fig.12.
This reaction on alkene generates vicinal diol and is a SYN-ADDITION reaction.
This is supported by the mechanism that the oxygen atoms of `OH` group in the diol formed are from the permanganate ions which add to the alkene molecule from the same side.
(ii) With `OsO_4` (Osmium tetraoxide) : See fig.13.
This is again a SYN-ADDITION reaction.
(iii) `text(With Hot)` `KMnO_4 :` Alkenes on reaction with hot alkaline `KMnO_4` give a mixture of carboxylic acid and ketones or only ketones or carboxylic acids.
`CH_2=CH_2 underset(KMnO_4)overset([O])-> 2 HCOOH underset(Delta)overset([O])-> 2CO_2 +2H_2O`
`CH_3 -CH =CH_2CH_3COOH +CO_2 +H_2O`
See fig.14.
`text(Addition of Oxygen :)` Alkenes on addition with oxygen in the presence of silver as catalyst at `570` `K` forms epoxide.
See fig.15.
`text(Substitution Reactions :)` Most alkenes contain not only the carbon-carbon double bond but also alkyl groups which have essentially alkane character. Hence besides the addition reactions which are characterstics of carbon-carbon double bonds, Alkenes may undergo the free radical substitution also which is a characteristic of alkanes.
`(a)` `text(Halogenation, Allylic Substitution)`
`H - underset(|)overset(|)C - overset(|)C - overset(|)C - X_2 underset(Delta)-> X - underset(|)overset(|)C - overset(|)C - overset(|)C - `
`CH_3 -CH = CH_2 underset(700^oC)overset(Cl_2)-> Cl - CH_2 -CH = CH_2`
See fig.16.
When we consider the molecule of propene
See fig.17.
The Site-I is the alkene site where due to the presence of reactive `pi`-bonds the reaction can take place at low temperature, in the absence of light and generally in liquid state. While site-II is the alkane site which requires high temperatures or U.V light. If we try to make the reaction possible at site-II, we must take the necessary condition of high temperature or U.V. radiations.
See fig.18.
`text(Mechanism)` :
lnilation `X_2 underset(U . V )oversettext(high temg.)-> 2X^(.)`
`text(Propogations :)`
`X^(.) +CH_3 -CH =CH_2 -> HX + overset(.)(CH_2) - CH =CH_2`
See fig.19.
Also `CH_3-CH -CH_2+SO_2 -> underset(|_(Cl))(CH_2) -CH _CH_2 +HCl +SO_2`
`CH_3 -CH -CH_2Me_5 COCl ->underset(|_(Cl))(CH_2) - CH -CH_2 +HCl`
`text(Addition of Carbenes :)` Alkenes react with diazomethane to form cyclic compounds.
See fig.20.
`text(Mechanism)` : See fig.21.
`text(Addition of Free Radicals :)` Analogous to free radical addition of `HBr` to alkenes, even `C Cl_4` or `CBr Cl_3`, or `CBr_2 Cl_2` can be added on to alkenes by using certain conditions favourable for free radical addition reaction
See fig.22.
`text(Mechanism)` :
Peroxide `->` Radical
Radical `+ C Cl_4 ->` Radical `Cl + overset(.)(CCl_3)`
`overset(.)(C Cl_3) + RCH =CH_2 -> R- overset(.)CH -CH_2 -C Cl_3`
See fig.23.
`text(Isomerization :)`
`CH_3CH_2CH_2CH =CH_3-CH_2 -CH = CH -CH_3CH_2 -CH = -CH_2`
`text(Diets Alder reaction :)` See fig.24.
Presence of electron donating groups on diene and electron withdrawing groups on dienophile favours the reaction.