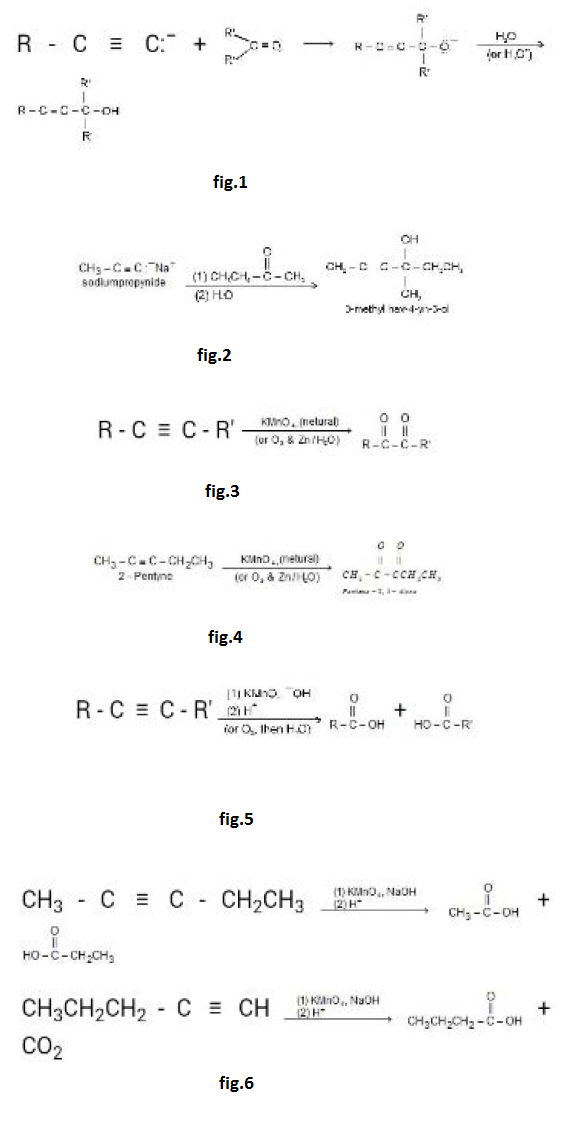
Introduction :

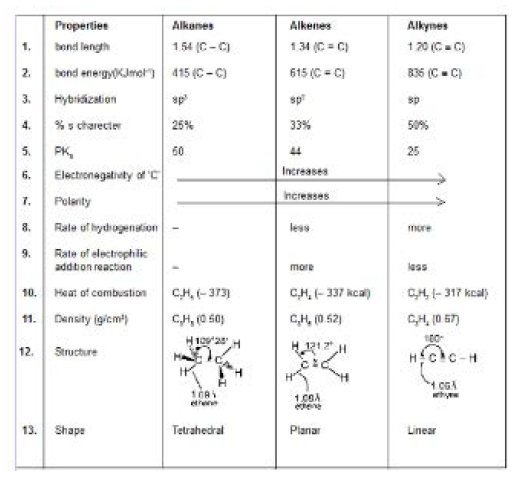
(i) Alkynes are hydrocarbons that contain carbon-carbon triple bond.
(ii) The general formula is : `C_nH_(2n-2)` (one triple bond)
(iii) The hybridization of carbon `(C equiv C)` in alkynes is carbon `sp`
(iv) Overlapping of these `sp` hybrid orbitals with each other and with the hydrogen `s` orbitals gives the sigma bond framework which is linear `(180^o)` structure.
(v) Two `pi` bonds result from overlap of the two remaining unhybridized `p` orbitals on each carbon atom. These orbitals overlap at right angles `(90^o)` to each other, forming one pi bond with electron density above and below the `C - C` sigma bond, and the other with electron density in front and in back of the sigma bond. This result in a cylindrical `pi` electron cloud around `sigma` bonded structure. See fig.
`text(Note :)` Any type of stereoisomerism does not arise in acetylenic bond due to linearity of `C equiv C` bond.
(ii) The general formula is : `C_nH_(2n-2)` (one triple bond)
(iii) The hybridization of carbon `(C equiv C)` in alkynes is carbon `sp`
(iv) Overlapping of these `sp` hybrid orbitals with each other and with the hydrogen `s` orbitals gives the sigma bond framework which is linear `(180^o)` structure.
(v) Two `pi` bonds result from overlap of the two remaining unhybridized `p` orbitals on each carbon atom. These orbitals overlap at right angles `(90^o)` to each other, forming one pi bond with electron density above and below the `C - C` sigma bond, and the other with electron density in front and in back of the sigma bond. This result in a cylindrical `pi` electron cloud around `sigma` bonded structure. See fig.
`text(Note :)` Any type of stereoisomerism does not arise in acetylenic bond due to linearity of `C equiv C` bond.