Physical Properties
(i) `text(Physical State :)` Lower alcohols are liquid at room temperature while higher ones are solid. They have distinct smell and burning taste.
(ii) `text(Boiling Point :)` The lower members like methanol, ethanol, 1 - propanol have higher boiling points. The boiling point rises as the molecular weights of the alcohol increases. It is quite evident that within the homologous series, the alcohols of normal chain show a rise in the boiling points with the increase in molecular weights. Like alkanes, the branched chain isomers of alcohol have lower boiling points. Thus among the four isomeric butyl alcohols, t - butyl has the lowest boiling points `(80^oC)`.
The order of boiling points of isomeric butyl alcohols is n - Butyl alcohol, `CH_3(CH_2)_3OH >` iso - butyl alcohol, `(CH_3)_2CHCH_2OH >` sec - butyl alcohol, `CH_3CH(OH)CH_2CH3 >` t - butyl alcohol, `(CH_3)_3COH` Compactness of t-butyl alcohol reduces the surface area and hence lowers the boiling point. The boiling point of alcohols are much higher than alkanes of comparable molecular weights. The high boiling point of the alcohols is due to hydrogen bonding by which the alcohol molecules remain associated in the liquid state.
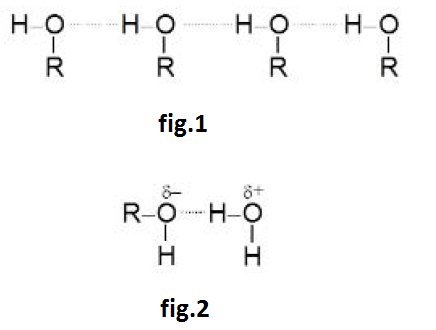
See fig.1.
The hydrogen of one (`-OH`) group forms a loose bond with the oxygen of `-OH` group of another molecule i.e. they remain in the molecular association through inter-molecular hydrogen bonding, which accounts for their high boiling points. But the hydrogen bonding is not so extensive as in water molecules. So, water boils at a higher temperature than methyl and ethyl alcohols.
(iii) `text(Solubility :)` The lower members of alcohols are highly soluble in water but as the size of the alkyl group increases, the solubility decreases. This phenomenon is common with other organic compounds having atleast one electronegative atom or group like ethers, aldehydes, ketones, acids, amides, sugars etc. and they all are soluble in water. The solubility of alcohols is attributed to its ability to form hydrogen bonds with water.
See fig.2.
But as the molecular weight increases, the solubility decreases. For example, methanol is infinitely soluble but only 0.6 g of n - hexyl alcohol dissolves in l 00 ml of water. I n general, organic compounds having atleast one electronegative element become gradually insoluble in water as the hydrocarbon chain increases. However, branching of the alcohol increases the solubility.
Thus, t - butyl alcohol is infi nitely soluble but 1 - butanol is slightly soluble in water. This is again due to the compactness of the molecule. Better and easy surrounding by water increases the solubility. Increase in the number of OH groups increases the solubility. For example, glycol (two OH groups) and glycerol (three OH groups) are more soluble in water than methanol and ethanol.
(ii) `text(Boiling Point :)` The lower members like methanol, ethanol, 1 - propanol have higher boiling points. The boiling point rises as the molecular weights of the alcohol increases. It is quite evident that within the homologous series, the alcohols of normal chain show a rise in the boiling points with the increase in molecular weights. Like alkanes, the branched chain isomers of alcohol have lower boiling points. Thus among the four isomeric butyl alcohols, t - butyl has the lowest boiling points `(80^oC)`.
The order of boiling points of isomeric butyl alcohols is n - Butyl alcohol, `CH_3(CH_2)_3OH >` iso - butyl alcohol, `(CH_3)_2CHCH_2OH >` sec - butyl alcohol, `CH_3CH(OH)CH_2CH3 >` t - butyl alcohol, `(CH_3)_3COH` Compactness of t-butyl alcohol reduces the surface area and hence lowers the boiling point. The boiling point of alcohols are much higher than alkanes of comparable molecular weights. The high boiling point of the alcohols is due to hydrogen bonding by which the alcohol molecules remain associated in the liquid state.
See fig.1.
The hydrogen of one (`-OH`) group forms a loose bond with the oxygen of `-OH` group of another molecule i.e. they remain in the molecular association through inter-molecular hydrogen bonding, which accounts for their high boiling points. But the hydrogen bonding is not so extensive as in water molecules. So, water boils at a higher temperature than methyl and ethyl alcohols.
(iii) `text(Solubility :)` The lower members of alcohols are highly soluble in water but as the size of the alkyl group increases, the solubility decreases. This phenomenon is common with other organic compounds having atleast one electronegative atom or group like ethers, aldehydes, ketones, acids, amides, sugars etc. and they all are soluble in water. The solubility of alcohols is attributed to its ability to form hydrogen bonds with water.
See fig.2.
But as the molecular weight increases, the solubility decreases. For example, methanol is infinitely soluble but only 0.6 g of n - hexyl alcohol dissolves in l 00 ml of water. I n general, organic compounds having atleast one electronegative element become gradually insoluble in water as the hydrocarbon chain increases. However, branching of the alcohol increases the solubility.
Thus, t - butyl alcohol is infi nitely soluble but 1 - butanol is slightly soluble in water. This is again due to the compactness of the molecule. Better and easy surrounding by water increases the solubility. Increase in the number of OH groups increases the solubility. For example, glycol (two OH groups) and glycerol (three OH groups) are more soluble in water than methanol and ethanol.