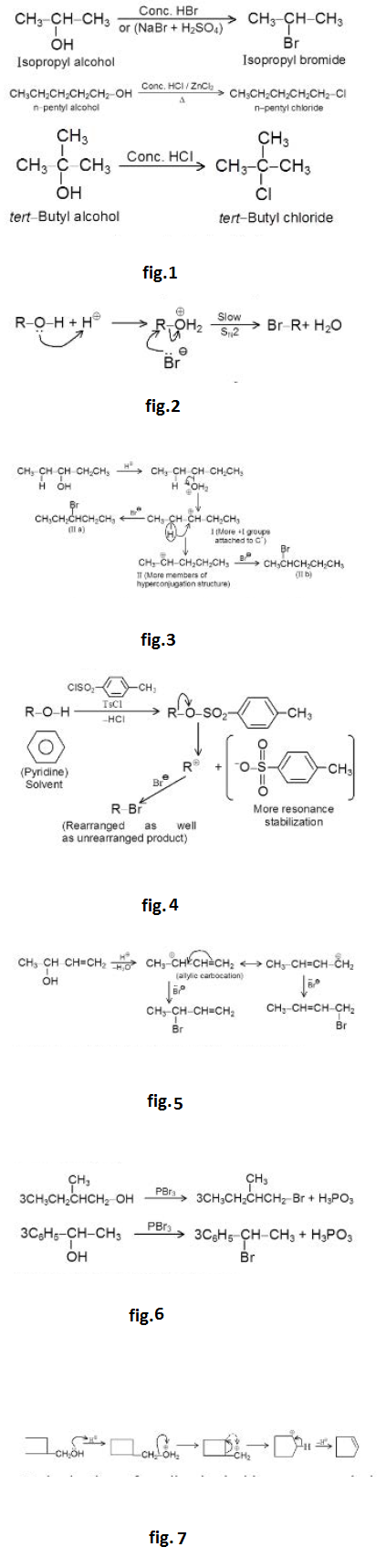
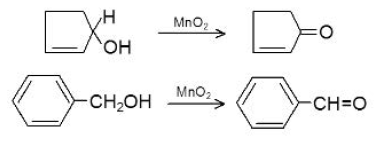
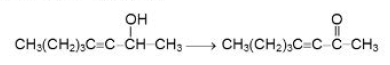
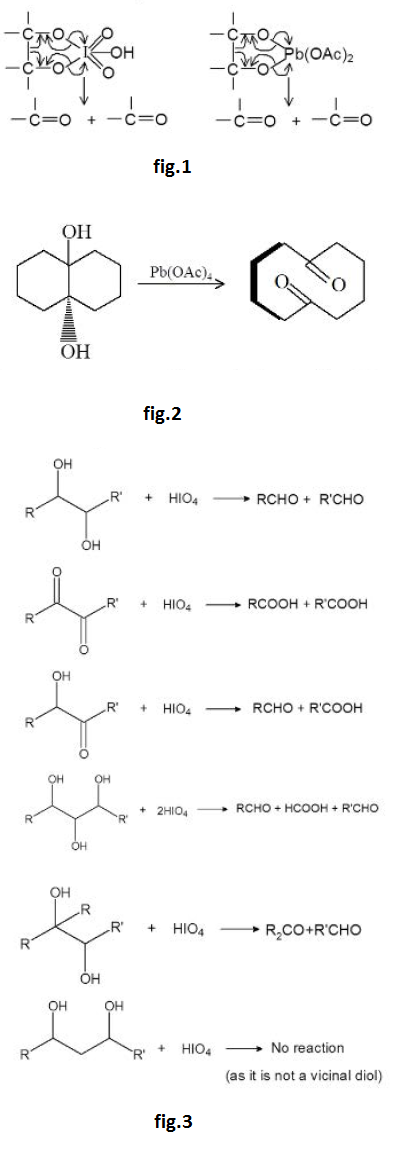
Reaction due to `O- H` Bond Cleavage :
`text(Reaction with Alkali Metals :)`
Active metals (`Na`, `K`, `Mg`, `Al` etc.) when treated with alcohols give hydrogen gas. In this reaction, order of reactivity of alcohols is `CH_3OH > 1^o > 2^o > 3o.` This reaction exhibits acidic character of alcohols.
`RO -H +Na -> RO^(-) Na^(+) +1/2 H_2`
In `-OH` group of alcohols, oxygen is more electronegative than hydrogen, this results in polarization of `O-H` bond due to which acidic nature arises in alcohols. Reaction of active metals with alcohols shows that alcohols are acidic in nature.
`undersettext(Stronger base)[RO^(-)Na^(+)] + undersettext(Stronger acid)(HOH) -> undersettext(Weaker base)(NaOH) + undersettext(Weaker acid)(ROH)`
The order of acidity for some compounds is
`H_2O > ROH > HC equiv CH > NH_3 > RH`
The order of basicity is
`R^(-) > NH_2^(-) > HC equiv C^(-) > OR^(-) > OH^(-)`
The above order is based on the reactions of alcohols with other species.
`C_2H_5OH + Na -> C_2H_5O^(⊖)Na^(oplus) + 1/2 H_2 uparrow`
`HC equiv C^(-)Na^(+) + RO -H -> HC equiv CH +RO^(-)Na^(+)`
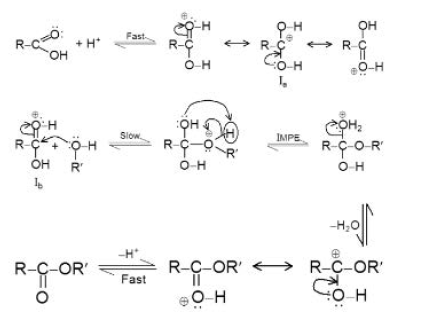
`text(Esterification :)` A direct reaction between a carboxylic acid and alcohol under the catalytic effect of sulphuric acid yields an ester. This is a reversible reaction and is known as the `" Fischer esterification ".` See fig.
Active metals (`Na`, `K`, `Mg`, `Al` etc.) when treated with alcohols give hydrogen gas. In this reaction, order of reactivity of alcohols is `CH_3OH > 1^o > 2^o > 3o.` This reaction exhibits acidic character of alcohols.
`RO -H +Na -> RO^(-) Na^(+) +1/2 H_2`
In `-OH` group of alcohols, oxygen is more electronegative than hydrogen, this results in polarization of `O-H` bond due to which acidic nature arises in alcohols. Reaction of active metals with alcohols shows that alcohols are acidic in nature.
`undersettext(Stronger base)[RO^(-)Na^(+)] + undersettext(Stronger acid)(HOH) -> undersettext(Weaker base)(NaOH) + undersettext(Weaker acid)(ROH)`
The order of acidity for some compounds is
`H_2O > ROH > HC equiv CH > NH_3 > RH`
The order of basicity is
`R^(-) > NH_2^(-) > HC equiv C^(-) > OR^(-) > OH^(-)`
The above order is based on the reactions of alcohols with other species.
`C_2H_5OH + Na -> C_2H_5O^(⊖)Na^(oplus) + 1/2 H_2 uparrow`
`HC equiv C^(-)Na^(+) + RO -H -> HC equiv CH +RO^(-)Na^(+)`
`text(Esterification :)` A direct reaction between a carboxylic acid and alcohol under the catalytic effect of sulphuric acid yields an ester. This is a reversible reaction and is known as the `" Fischer esterification ".` See fig.