Chemical Reaction of `H_2SO_4` :
(i) lt has greater affinity for water. Hence is used for drying of all gases except ammonia. lt is also employed as dehydrating agent.
(a) `C_(12)H_(22)O_(11) overset(H_2SO_4)-> 12C + 11 H_2O`
This called as charring of sugars.
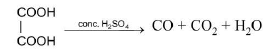
(b) See fig.
(c) Chloro benzene+ Chloral `overset(H_2SO_4)-> ` DDTE
(ii) Action with potassium ferrocyanide :
`K_4Fe(CN)_6 + 6H_2SO_4 + 6H_2O -> 2K_2SO_4 + FeSO_4 + 3(NH_4)_2SO_4 + 6CO`
(a) `C_(12)H_(22)O_(11) overset(H_2SO_4)-> 12C + 11 H_2O`
This called as charring of sugars.
(b) See fig.
(c) Chloro benzene+ Chloral `overset(H_2SO_4)-> ` DDTE
(ii) Action with potassium ferrocyanide :
`K_4Fe(CN)_6 + 6H_2SO_4 + 6H_2O -> 2K_2SO_4 + FeSO_4 + 3(NH_4)_2SO_4 + 6CO`