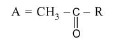Fluorine (`F_2`) :
Modern method of Isolation :
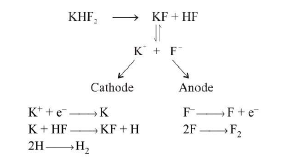
In this method `F_2` is prepared by the electrolysis of fused fluoride, (`KHF_2`) potassium hydrogen fluoride. The electrolytic cells are made of `Cu`, `Ni` or monel metal. Reaction in the electrolytic cell : See fig.
(i) `text(Uses :)` It is used in the preparation of fluorine compound such as.
(a) Freon: Freon - 12 i.e. `CF_2Cl_2` as used in refrigeration and air conditioning in place of `NH_3` and
(b) Teflon : `(- F_2C - CF_2)_n`. It is a new plastic.
In this method `F_2` is prepared by the electrolysis of fused fluoride, (`KHF_2`) potassium hydrogen fluoride. The electrolytic cells are made of `Cu`, `Ni` or monel metal. Reaction in the electrolytic cell : See fig.
(i) `text(Uses :)` It is used in the preparation of fluorine compound such as.
(a) Freon: Freon - 12 i.e. `CF_2Cl_2` as used in refrigeration and air conditioning in place of `NH_3` and
(b) Teflon : `(- F_2C - CF_2)_n`. It is a new plastic.