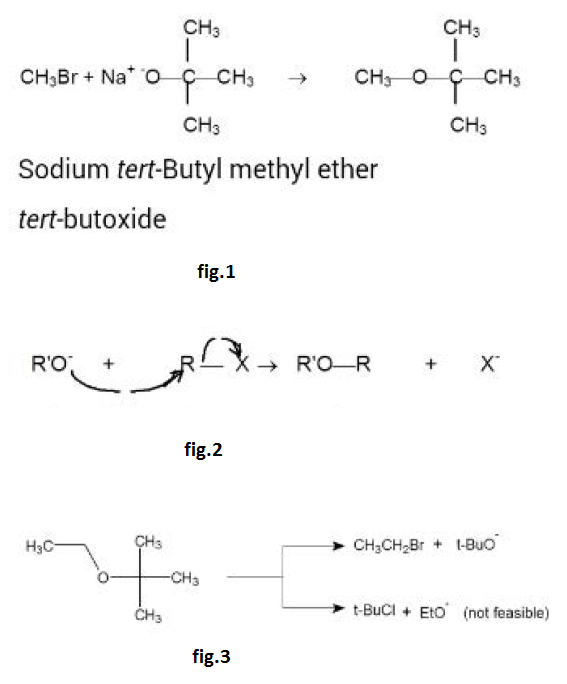
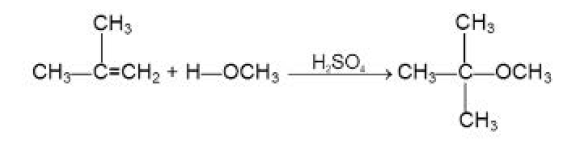
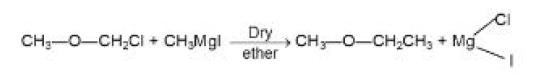
From Alcohols :
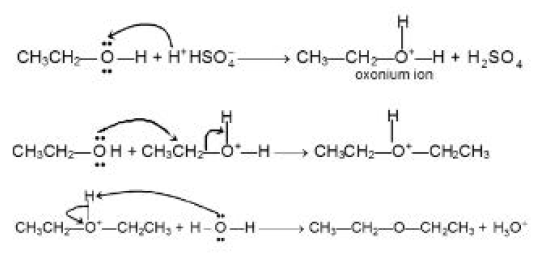
Ethers may be prepared by dehydration of alcohols either in the presence of acid or heated alumina.
(a) Acid -catalysed dehydration :
The yield of alcohol however depends on the nature of alcohols (`1^o` ,`2^o` or `3^o`) `2^o` alcohol mainly gives alkenes with low yields of ethers whereas `3^o` exclusively gives alkenes.
Order of dehydration of alcohol or formation of ethers : `1^o > 2^o > 3^o`
`CH_3CH_2OH underset(140^oC)overset(H_2SO_4)-> undersettext(Ether)(CH_3CH_2 -O - CH_2CH_3)`
`text(Mechanism of)` `S_N2` : See fig.
Catalytic dehydration : Dehydration of alcohols to ethers can also be achieved by passing the vapours of an alcohol over heated alumnia at `523` `K`.
e.g., `CH_3CH_2-OH + H-OCH_2CH_3 underset(523 K)overset(Al_2O_3)-> CH_3CH_2-O-CH_2CH_3 + H_2O`
(b) By the action of diazomethane on alcohols - Methyl ethers can also be prepared by action of `CH_2N_2` on alcohols in presence of fluoroboric acid (`HBF_4`) as catalyst.
`CH_3CH_2OH + CH_2N_2 overset(HBF_4)-> CH_3CH_2-O-CH_3 + N_2`
(a) Acid -catalysed dehydration :
The yield of alcohol however depends on the nature of alcohols (`1^o` ,`2^o` or `3^o`) `2^o` alcohol mainly gives alkenes with low yields of ethers whereas `3^o` exclusively gives alkenes.
Order of dehydration of alcohol or formation of ethers : `1^o > 2^o > 3^o`
`CH_3CH_2OH underset(140^oC)overset(H_2SO_4)-> undersettext(Ether)(CH_3CH_2 -O - CH_2CH_3)`
`text(Mechanism of)` `S_N2` : See fig.
Catalytic dehydration : Dehydration of alcohols to ethers can also be achieved by passing the vapours of an alcohol over heated alumnia at `523` `K`.
e.g., `CH_3CH_2-OH + H-OCH_2CH_3 underset(523 K)overset(Al_2O_3)-> CH_3CH_2-O-CH_2CH_3 + H_2O`
(b) By the action of diazomethane on alcohols - Methyl ethers can also be prepared by action of `CH_2N_2` on alcohols in presence of fluoroboric acid (`HBF_4`) as catalyst.
`CH_3CH_2OH + CH_2N_2 overset(HBF_4)-> CH_3CH_2-O-CH_3 + N_2`