Reactions of Ethers : Cleavage by Acids :
Ethers are generally less reactive and react only with acids. The reactive sites in ethers are :
i) `C-H` bond
ii) `-O-` group of ether bond
Ethers resist the attack of nucleophiles and bases. However, they are very good solvents in many organic reactions due to their ability to solvate cations by donating the electron pair from oxygen atom.
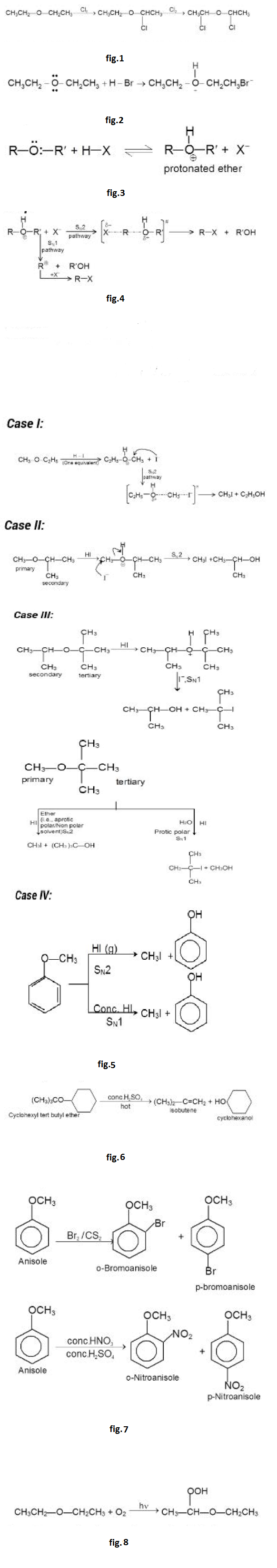
(a) `text(Halogenation of Ethers :)` Ethers undergo halogenation in dark to give halogenated ethers. The hydrogen atom attached to the `C` atom directly linked to oxygen atom is replaced by halogens. See fig.1.
(b) `text(Ethers as Base :)` `O` atom of ethers makes them basic. They react with a proton donor to give oxonium salts. See fig.2.
(c) `text(Reaction with Acids :)`
(i) Cold conc. `HI//HBr` - Ether undergoes cleavage with cold conc. solution of `HI//HBr` to give a mixture of alcohol and iodides. The smaller of alkyl groups goes with the halide and larger group forms alcohols.
`R- O - R + undersettext(cold)(HI) -> R- OH + RI (R < R)`
(ii) Hot conc. `HI//HBr` - On heating ethers with conc. `HI//HBr` ethers gives two molar equivalents of halides.
`R- O - R + undersettext(hot conc.)(HI) -> RI + R'I + H_2O`
In the first step, ether is protonated by `HX` to give protonated ether. In the second step, halide ion acts as nucleophile and attacks protonated ether to undergo cleavage. This step is favoured because the leaving group (alcohol) is weakly basic.
`text(Step I)` : See fig.3.
`text(Step II)` : See fig.4.
Reaction in second step can take the direction of `S_N 1` or `S_N2` pathway, depending upon the conditions employed and the structure of ether. When both the alkyl groups are methyl or `1^o`, it will follow `S_N2` reaction and when atleast one of the alkyl group is `3^o`, the reaction follows `S_N 1` pathway. For all other cases, it can undergo `S_N 1` or `S_N2` pathway, depending upon the reaction conditions.
For example, See fig.5.
`text(Hint :)`
a) Methyl cation is stable than phenyl cations.
b) Hot conc. `H_2SO_4` - secondary and tertiary ethers react with to give a mixture of alcohols and alkenes.
`(CH_3)_3CO - CH_3 undersettext(hot)overset[text(conc.) H_2 SO_4]-> (CH_3)_2 - C= CH_2 + CH_3OH`
See fig.6.
`text(Example :)`
`undersettext(diethyl ether)(CH_3CH_2 - O - CH_2CH_3) + undersettext(conc.)(H_2SO_4) -> undersettext(ethyl alcohal)(CH_3CH_2 -OH) +undersettext(ethyldrogen sulphate)(CH_3CH_2 -OSO_2 OH)`
(d) Reaction with acid chlorides and anhydrides : Acid chlorides react with ethers when heated in the presence of anhyd. `ZnCl_2` or `AlCl_3` to form chloride and esters.
`undersettext(diethyl ether)(C_2H_5 - O -C_2H_5) + undersettext(Acetyl chloride)(CH_3COCl) underset(Delta)overset[text(anhyd.) ZnCl_2]-> undersettext(ethyl chloride) (C_2H_5Cl) +undersettext(ethylacetate)(CH_3COOC_2H_5)`
Anhydride react with ethers to give only esters
`undersettext(diethylether)[(C_2H_5)_2O] + undersettext(Acetic anhydride)[(CH_3CO)_2O] underset(Delta)overset[text(anhy. AlCl_3)]-> undersettext(Ethylacetate)(2CH_3COOC_2H_5)`
Electrophilic Substitution Reactions : See fig.7.
(e) Action of air and light - Reaction involving alkyl group leads to the formation of peroxides. See fig.8.
It is a free radical reaction and oxidation occurs at the `C` atom next to the ethereal oxygen to form hydroperoxides.
i) `C-H` bond
ii) `-O-` group of ether bond
Ethers resist the attack of nucleophiles and bases. However, they are very good solvents in many organic reactions due to their ability to solvate cations by donating the electron pair from oxygen atom.
(a) `text(Halogenation of Ethers :)` Ethers undergo halogenation in dark to give halogenated ethers. The hydrogen atom attached to the `C` atom directly linked to oxygen atom is replaced by halogens. See fig.1.
(b) `text(Ethers as Base :)` `O` atom of ethers makes them basic. They react with a proton donor to give oxonium salts. See fig.2.
(c) `text(Reaction with Acids :)`
(i) Cold conc. `HI//HBr` - Ether undergoes cleavage with cold conc. solution of `HI//HBr` to give a mixture of alcohol and iodides. The smaller of alkyl groups goes with the halide and larger group forms alcohols.
`R- O - R + undersettext(cold)(HI) -> R- OH + RI (R < R)`
(ii) Hot conc. `HI//HBr` - On heating ethers with conc. `HI//HBr` ethers gives two molar equivalents of halides.
`R- O - R + undersettext(hot conc.)(HI) -> RI + R'I + H_2O`
In the first step, ether is protonated by `HX` to give protonated ether. In the second step, halide ion acts as nucleophile and attacks protonated ether to undergo cleavage. This step is favoured because the leaving group (alcohol) is weakly basic.
`text(Step I)` : See fig.3.
`text(Step II)` : See fig.4.
Reaction in second step can take the direction of `S_N 1` or `S_N2` pathway, depending upon the conditions employed and the structure of ether. When both the alkyl groups are methyl or `1^o`, it will follow `S_N2` reaction and when atleast one of the alkyl group is `3^o`, the reaction follows `S_N 1` pathway. For all other cases, it can undergo `S_N 1` or `S_N2` pathway, depending upon the reaction conditions.
For example, See fig.5.
`text(Hint :)`
a) Methyl cation is stable than phenyl cations.
b) Hot conc. `H_2SO_4` - secondary and tertiary ethers react with to give a mixture of alcohols and alkenes.
`(CH_3)_3CO - CH_3 undersettext(hot)overset[text(conc.) H_2 SO_4]-> (CH_3)_2 - C= CH_2 + CH_3OH`
See fig.6.
`text(Example :)`
`undersettext(diethyl ether)(CH_3CH_2 - O - CH_2CH_3) + undersettext(conc.)(H_2SO_4) -> undersettext(ethyl alcohal)(CH_3CH_2 -OH) +undersettext(ethyldrogen sulphate)(CH_3CH_2 -OSO_2 OH)`
(d) Reaction with acid chlorides and anhydrides : Acid chlorides react with ethers when heated in the presence of anhyd. `ZnCl_2` or `AlCl_3` to form chloride and esters.
`undersettext(diethyl ether)(C_2H_5 - O -C_2H_5) + undersettext(Acetyl chloride)(CH_3COCl) underset(Delta)overset[text(anhyd.) ZnCl_2]-> undersettext(ethyl chloride) (C_2H_5Cl) +undersettext(ethylacetate)(CH_3COOC_2H_5)`
Anhydride react with ethers to give only esters
`undersettext(diethylether)[(C_2H_5)_2O] + undersettext(Acetic anhydride)[(CH_3CO)_2O] underset(Delta)overset[text(anhy. AlCl_3)]-> undersettext(Ethylacetate)(2CH_3COOC_2H_5)`
Electrophilic Substitution Reactions : See fig.7.
(e) Action of air and light - Reaction involving alkyl group leads to the formation of peroxides. See fig.8.
It is a free radical reaction and oxidation occurs at the `C` atom next to the ethereal oxygen to form hydroperoxides.