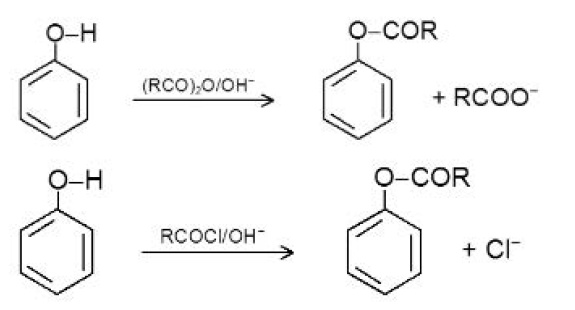
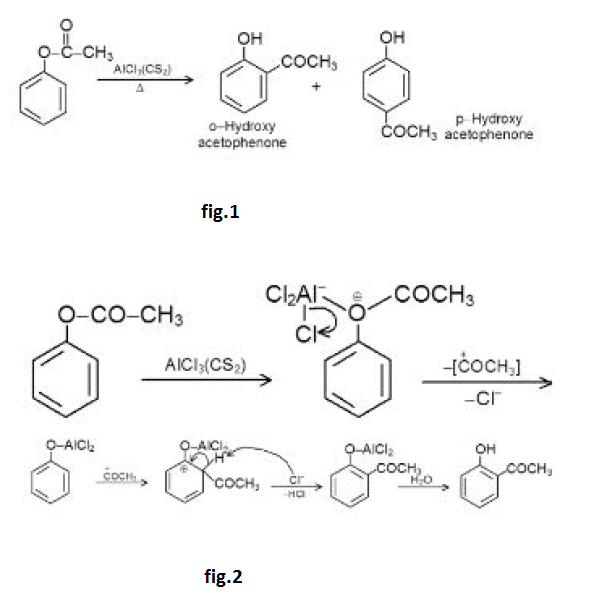
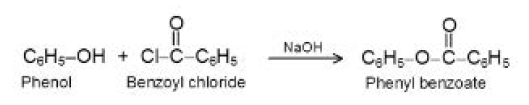
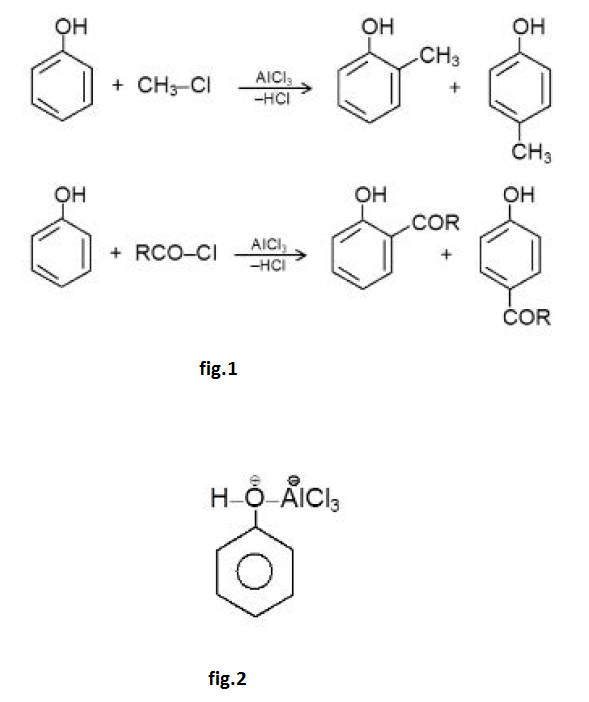
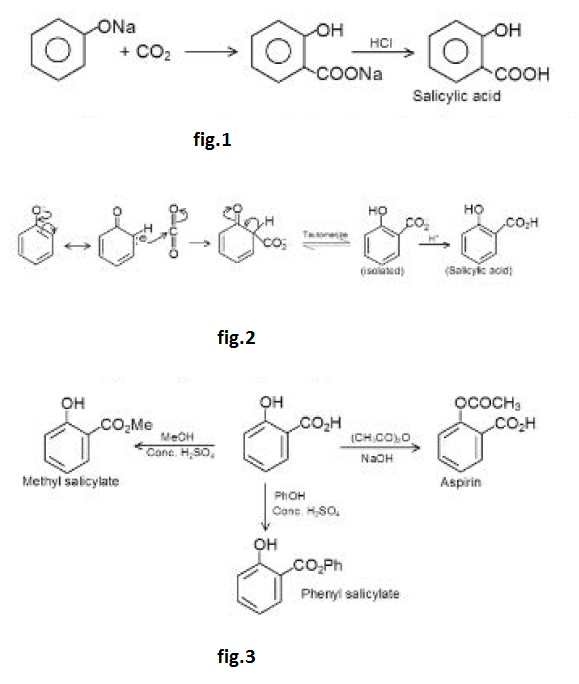
Acidity of Phenols :
Phenols are weak acids (`pK_a = 10`). They form salts with aqueous `NaOH` but not with aqueous `NaHCO_3`.
Although Phenols are structurally similar to alcohols, they are much stronger acids. But phenol is a weak acid when compared to a carboxyllic acid, such as acetic acid (`pK_a = 4.7447`).
The greater acidity of phenol owes itself primarily to an electrical charge distribution in phenols that causes the `-OH` oxygen to be more positive; therefore the proton is held less strongly.
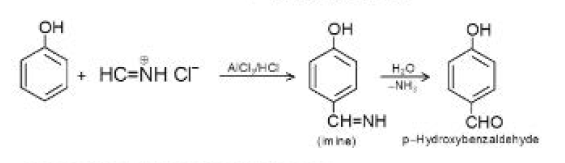
The factor influencing the electron distribution may be the contributions to the overall resonance hybrid of phenol made by the resonance structures shown below. The effect of these structures is to withdraw electrons from the hydroxyl group and to make the oxygen positive. See fig.1.
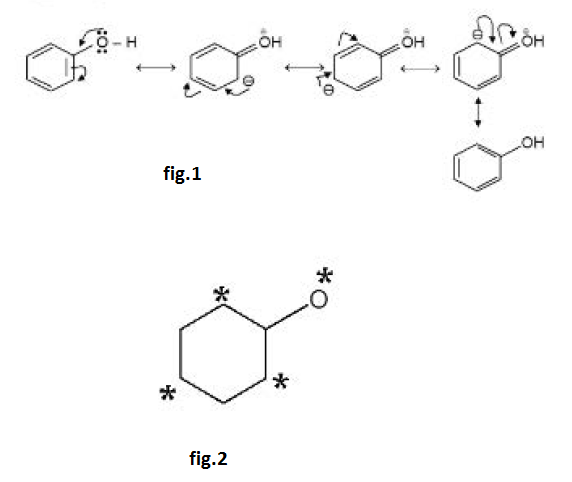
The considerably greater acid strength of `PhOH (pK_a = 10)` than that of `ROH (pK_a = 18)` can be accounted for as the negative charge on the alkoxide anion, `RO^(-)`, cannot be delocalized, but on `PhO^(-)` the negative charge is delocalized to the ortho and para ring positions as indicated by the starred sites in the resonance hybrid. See fig.2.
`PhO^(-)` is therefore a weaker base than `RO^(-)` and `PhOH` is a stronger acid the effect of
a) electron - attracting and
b) electron - releasing substituents on the acid strength of phenols
Electron-attracting substituents disperse negative charges and therefore stabilize `ArO^(-)` and increase acidity of `ArOH`. Electron- releasing substituents concentrate the negative charge on `O` destabilizes `ArO^(-)` and decreases acidity of `ArOH`.
In terms of resonance and inductive effects we can account for the following relative acidities.
a) `p-O_2NC_6H_4OH > m- O_2NC_6H_4OH > C_6H_5OH`
b) `m- ClC_6H_4OH > p-ClC_6H_4OH > C_6H_5OH`
a) The- `NO_2` is electron-withdrawing and acid strengthening. Its resonance effect, which occurs only from para and ortho positions,
predominates over its inductive effect, which occurs also from the meta position.
b) `Cl` is electron-withdrawing by inductive effect. This effect diminishes with increasing distance between `Cl` and `OH`. The meta is closer than the para positions and `m-Cl` is more acid - strengthening than the `p-Cl`. Other substituents in this category are `F`, `Br`, `I`, `text( )^(+)NR_3`.
Although Phenols are structurally similar to alcohols, they are much stronger acids. But phenol is a weak acid when compared to a carboxyllic acid, such as acetic acid (`pK_a = 4.7447`).
The greater acidity of phenol owes itself primarily to an electrical charge distribution in phenols that causes the `-OH` oxygen to be more positive; therefore the proton is held less strongly.
The factor influencing the electron distribution may be the contributions to the overall resonance hybrid of phenol made by the resonance structures shown below. The effect of these structures is to withdraw electrons from the hydroxyl group and to make the oxygen positive. See fig.1.
The considerably greater acid strength of `PhOH (pK_a = 10)` than that of `ROH (pK_a = 18)` can be accounted for as the negative charge on the alkoxide anion, `RO^(-)`, cannot be delocalized, but on `PhO^(-)` the negative charge is delocalized to the ortho and para ring positions as indicated by the starred sites in the resonance hybrid. See fig.2.
`PhO^(-)` is therefore a weaker base than `RO^(-)` and `PhOH` is a stronger acid the effect of
a) electron - attracting and
b) electron - releasing substituents on the acid strength of phenols
Electron-attracting substituents disperse negative charges and therefore stabilize `ArO^(-)` and increase acidity of `ArOH`. Electron- releasing substituents concentrate the negative charge on `O` destabilizes `ArO^(-)` and decreases acidity of `ArOH`.
In terms of resonance and inductive effects we can account for the following relative acidities.
a) `p-O_2NC_6H_4OH > m- O_2NC_6H_4OH > C_6H_5OH`
b) `m- ClC_6H_4OH > p-ClC_6H_4OH > C_6H_5OH`
a) The- `NO_2` is electron-withdrawing and acid strengthening. Its resonance effect, which occurs only from para and ortho positions,
predominates over its inductive effect, which occurs also from the meta position.
b) `Cl` is electron-withdrawing by inductive effect. This effect diminishes with increasing distance between `Cl` and `OH`. The meta is closer than the para positions and `m-Cl` is more acid - strengthening than the `p-Cl`. Other substituents in this category are `F`, `Br`, `I`, `text( )^(+)NR_3`.