Oxy Acids of Halogens :
(i) Fluorine does not form any oxy-acids because it is more electronegative than oxygen.
(ii) Other halogens form four series of oxy acids with formulae
`HXO` -> Hypohalous
`HXO_2 ->` Halous
`HXO_3 ->` Halic
`HXO_4 ->` Perhalic acids or Halic (I), Halic (III), Halic (V) and Halic (VII)
Some important general trends in Oxy-acids of Halogens
(i) In oxy-acids, hydrogen is present as `-OH` group.
(ii) All the hypohalous acids (`HXO`) are unstable and readily form `HXO`. Among these the relative order of stability is `HClO > HBrO > HIO`
(iii) In halic acids `(HXO_3)`, iodic acid is the most stable.
(iv) Thermal stability-
Thermal stability or oxidation state of halogens & No. of oxygen atoms.
(a) The thermal stability of both the acids and their salts increases with the increasing oxidation state of the halogen or with the increase in the number of oxygen atoms i.e. stability of the oxy halide anion increases from `ClO^(-), ClO_2^(-) , ClO_3^(-) , ClO_4^(-)`
(b) This is due to the fact with the increasing number of oxygen atoms in the series, the no. of electron involved in forming `sigma` and `pi` bonds increases.
(c) Thus in the most stable perchlorate ion, `ClO_4^(-)`, all the valence orbitals and electron of chlorine atom are involved in the formation of bonds.
(d) The stability of perchlorate ion, `ClO_4^(-)` may also be said due to greater multiplicity of the `Cl-O` bond.
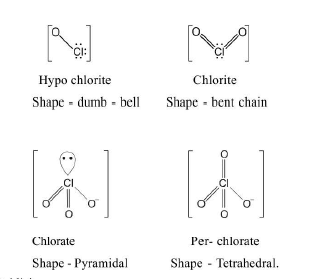
See fig.1.
(v) Oxidising power - Hypochtorites are the strongest oxidising agents.
(vi) Relative acidity `prop` oxidation no.
`HClO_4 > HClO_3 > HClO_2 > HClO`
`text(Note :)` (i) ln all these acids and salts halogen is in `sp^3` hybridised state.
(ii) Stronger the acid, the weaker will be its conjugate base and vice-versa.
`ClO_4^(-) < ClO_3^(-) < ClO_2^(-) < ClO^(-)` (relative basic character)
Thus `ClO_4^-` is the weakest base and `HClO_4` (conjugate acid of `ClO_4^(-)`)is the strongest acid.l
`underset(<-)(ClO_4^(-) < ClO_3^(-) < ClO_2^(-) < ClO^(-))`
Relative stability of `Cl-O` bonds
(ii) Other halogens form four series of oxy acids with formulae
`HXO` -> Hypohalous
`HXO_2 ->` Halous
`HXO_3 ->` Halic
`HXO_4 ->` Perhalic acids or Halic (I), Halic (III), Halic (V) and Halic (VII)
Some important general trends in Oxy-acids of Halogens
(i) In oxy-acids, hydrogen is present as `-OH` group.
(ii) All the hypohalous acids (`HXO`) are unstable and readily form `HXO`. Among these the relative order of stability is `HClO > HBrO > HIO`
(iii) In halic acids `(HXO_3)`, iodic acid is the most stable.
(iv) Thermal stability-
Thermal stability or oxidation state of halogens & No. of oxygen atoms.
(a) The thermal stability of both the acids and their salts increases with the increasing oxidation state of the halogen or with the increase in the number of oxygen atoms i.e. stability of the oxy halide anion increases from `ClO^(-), ClO_2^(-) , ClO_3^(-) , ClO_4^(-)`
(b) This is due to the fact with the increasing number of oxygen atoms in the series, the no. of electron involved in forming `sigma` and `pi` bonds increases.
(c) Thus in the most stable perchlorate ion, `ClO_4^(-)`, all the valence orbitals and electron of chlorine atom are involved in the formation of bonds.
(d) The stability of perchlorate ion, `ClO_4^(-)` may also be said due to greater multiplicity of the `Cl-O` bond.
See fig.1.
(v) Oxidising power - Hypochtorites are the strongest oxidising agents.
(vi) Relative acidity `prop` oxidation no.
`HClO_4 > HClO_3 > HClO_2 > HClO`
`text(Note :)` (i) ln all these acids and salts halogen is in `sp^3` hybridised state.
(ii) Stronger the acid, the weaker will be its conjugate base and vice-versa.
`ClO_4^(-) < ClO_3^(-) < ClO_2^(-) < ClO^(-)` (relative basic character)
Thus `ClO_4^-` is the weakest base and `HClO_4` (conjugate acid of `ClO_4^(-)`)is the strongest acid.l
`underset(<-)(ClO_4^(-) < ClO_3^(-) < ClO_2^(-) < ClO^(-))`
Relative stability of `Cl-O` bonds