Acidity of Carboxylic Acids :
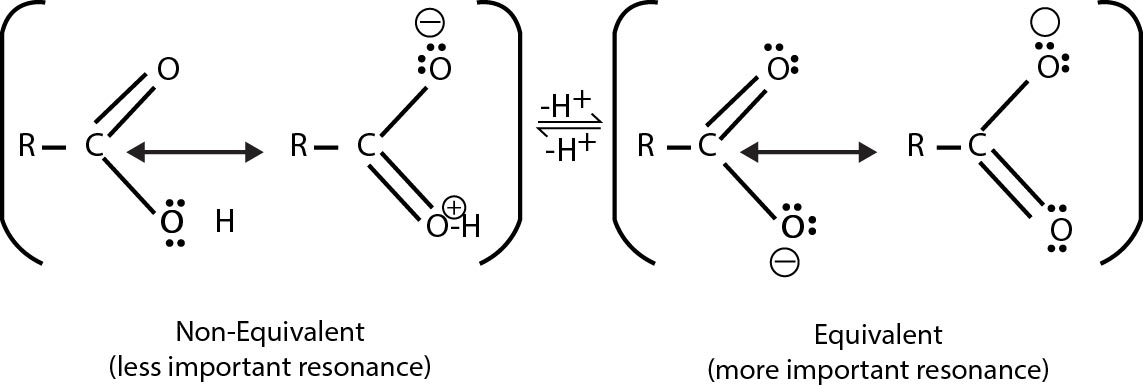
The acidity of a carboxylic acid is due to the resonance stabilization of its anion. See fig.
Because of the resonance both the carbon oxygen bond in the carboxylate anion have identical bond length. In the carboxylic acid, these bond lengths are no longer identical.
The acidity of carboxylic acid depends very much on the substituent attached to `-CO OH` group. Since acidity is due to the resonance stabilization of anion, substituent causing stabilization of anion increases acidity whereas substituent causing destabilization of anion decrease acidity. For example, electron withdrawing group disperses the negative charge of the anion and hence makes it more stable causing increase in the acidity of the corresponding acid, on the other hand, electron-releasing group increases the negative charge on the anion and hence makes it less stable causing the decrease in the acidity. In the light of this, the following are the orders of a few substituted carboxylic acids.
a) Increase in the number of Halogen atoms on `alpha`- position increases the acidity. eg-
`Cl_2C CO OH > Cl_2CHOOH > ClCH_2COOH > CH_3COOH`
b) Increase in the distance of Halogen from `-COOH` decreases the acidity e.g.
`CH_3- CH_2- CHCl - COOH > CH_3- CHCl - CH_2- COOH > ClCH_2- CH_2- CH_2- COOH`
This is due to the fact that inductive effect decreases with increasing distance.
c) Increase in the electronegativity of halogen increases the acidity.
`FCH_2COOH > BrCH_2cOOH > ICH_2COOH`
Because of the resonance both the carbon oxygen bond in the carboxylate anion have identical bond length. In the carboxylic acid, these bond lengths are no longer identical.
The acidity of carboxylic acid depends very much on the substituent attached to `-CO OH` group. Since acidity is due to the resonance stabilization of anion, substituent causing stabilization of anion increases acidity whereas substituent causing destabilization of anion decrease acidity. For example, electron withdrawing group disperses the negative charge of the anion and hence makes it more stable causing increase in the acidity of the corresponding acid, on the other hand, electron-releasing group increases the negative charge on the anion and hence makes it less stable causing the decrease in the acidity. In the light of this, the following are the orders of a few substituted carboxylic acids.
a) Increase in the number of Halogen atoms on `alpha`- position increases the acidity. eg-
`Cl_2C CO OH > Cl_2CHOOH > ClCH_2COOH > CH_3COOH`
b) Increase in the distance of Halogen from `-COOH` decreases the acidity e.g.
`CH_3- CH_2- CHCl - COOH > CH_3- CHCl - CH_2- COOH > ClCH_2- CH_2- CH_2- COOH`
This is due to the fact that inductive effect decreases with increasing distance.
c) Increase in the electronegativity of halogen increases the acidity.
`FCH_2COOH > BrCH_2cOOH > ICH_2COOH`