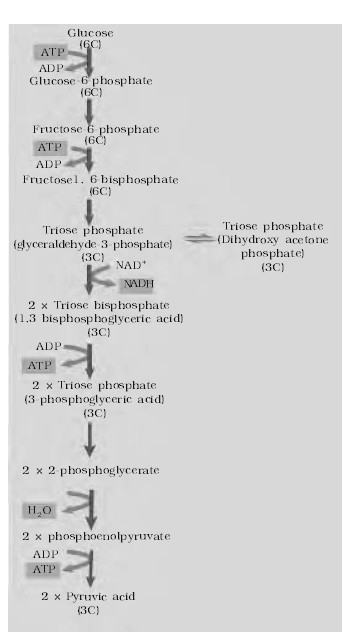
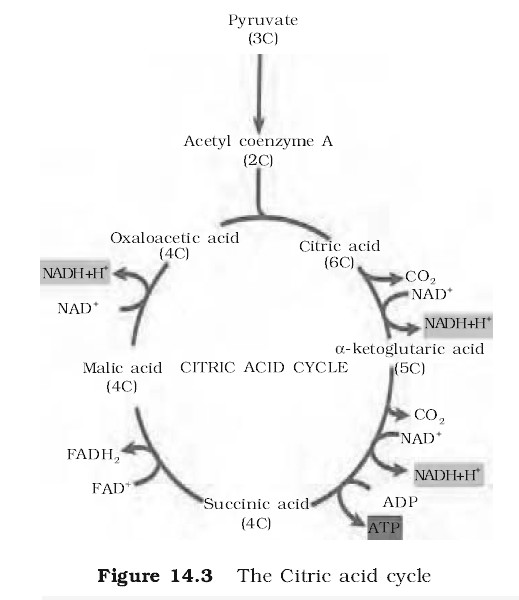
The electron transmitter system is also called electron transport chain (ETC), or cytochrome system (CS), as four out of these seven carriers are cytochrome. It is the major source of cells energy, in the respiratory breakdown of simple carbohydrates intermediates like phosphoglyceraldehyde, pyruvic acid, isocitric acid, ketoglutaric acid, succinic acid and malic acid are oxidised. The oxidation in all these brought about by the removal of a pair of hydrogen atoms (2H) from each of them. This final stage of respiration is carried out in ETS, located in the inner membrane of mitochondria (in prokaryotes the ETS is located in mesosomes of plasma membrane). The system consists of series of precisely arranged seven electron carriers (coenzyme) in the inner membrane of the mitochondrion, including the folds or cristae of this membrane. These seven electron-carriers function in a specific sequence and are : Nicotinamide adenine dinucleotide (NAD), Flavin mononucleotide (FMN), Flavin adenine dinucleotide (FAD), Co-enzyme-Q or ubiquinone, Cytochrome-b, Cytochrome-c, Cytochrome-a and Cytochrome-a3
# Five complex theory : According to Hatefi, (1976), Complex I to Complex IV are related to the electron transport.
Complex V related to mainly with ATP synthesis, so it is called ATPase /ATP syntheses complex.
The head piece (F1) of the oxysome consists of 5 hydrophobic subunits ( ), which are responsible for ATPase functioning.
The stalk (F0) contain F5 (oligomycin sensitivity conferring protein) i.e. CSCP and F6. F0 are related to the proton channel and embeded fully in thickness of inner mitochondrial membrane.
Five complex i.e. I, II, III, IV, V, have been isolated from mitochondrial membrane by chemical treatment.
Complex I : NADH/NADPH : CoQ reductase
Complex II : Succinate : CoQ reductase
Complex III : Reduced CoQ (CoQH2) : cytochrome C reductase
Complex IV : Cytochrome C oxidase
Complex V : ATPase
# The first carrier in the chain is a flavoprotein which is reduced by NADH2. Coenzyme passes these electron to the cytochromes arranged in the sequence of b-c-a-a3, finally pass the electron to molecular oxygen. In this transport, the electrons tend to flow from electro-negative to electro-positive system, so there is a decrease in free energy and some energy is released so amount of energy with the electrons goes on decreasing. During electron-transfer, the electron-donor gets oxidised, while electron-acceptor gets reduced so these transfers involve redox-reaction and are catalysed by enzymes, called reductases. Oxidation and reduction are complimentary. This oxidation-reductiion reaction over the ETC is called biological oxidation.
During the electron transfers, the energy released at some steps is so high that ATP is formed by the phosphorylation of ADP in the presence of enzyme ATP synthetase present in the head of F1-particles present on the mitochondrial crista. This process of ATP synthesis during oxidation of coenzyme is called oxidative phosphorylation, so ETS is also called oxidative phosphorylation pathways.
From the cytochrome a3, two electrons are received by oxygen atom which also receives two proton (H+) from the mitochondrial matrix to form water molecule. So the final acceptor electrons is oxygen. So the reaction H2 + O2 ---> H2O (called metabolic water) is made to occur in many steps through ETC, so the most of the energy can be derived into a storage and usable form.
# Two route systems of ETC : The pairs of hydrogen atoms from respiratory intermediates are received either by NAD+ or FAD coenzymes which becomes reduced to NADH2 and FADH2. These reduced coenzyme pass the electrons on to ETC. Thus, regeneration of NAD+ or FAD takes place in ETC. There are two routes ETC :
- (a) Route 1 : NADH2 passes their electrons to Co-Q through FAD . In route 1 FAD is the first electron carrier. 3 ATP molecules are produced during the transfer of electron on following steps :
NAD to FAD
Cyt b to Cyt c and
Cyt a to Cyt a3
- (b) Route 2 : FADH2 passes their electron directly to FAD. 2 ATP molecules are produced during the transfer of electron on following steps.
Cyt b to Cyt c and
Cyt a to Cyt a3
# Development of proton gradient : At each step of ETC, the electron- acceptor has a higher electron –affinity than the electron-donor. The energy from electron-transport is used to move the proton (H+) from the mitochondrial matrix to inter-membranous or outer chamber. Three pairs of protons are pushed to outer chamber during the movement of electrons along route I while two pairs of protons are moved to outer chamber during the movement of electrons along route–II.
This generates a pH-gradient across the inner mitochondrial membrane with protons (H+) concentration higher in the outer chamber than in the mitochondrial matrix. This difference in H+ concentration across the inner mitochondrial membrane is called proton-gradient
( pH). Due to proton gradient, an electrical potential is developed across the inner mitochondrial membrane as the matrix is now electronegative with respect to the intermembranous (outer) chamber. The proton gradient and membrane electric potential collectively called proton motive force.
# (b) Proton flow : Due to proton-gradient, the protons returns to the matrix while passing through proton channel of F0-F1 ATPase. This proton gradient activates the enzyme ATP synthetase or F0 – F1 ATPase
ATP synthetase controls the formation of ATP from ADP and inorganic phosphate in the presence of energy.
The electron transmitter system is also called electron transport chain (ETC), or cytochrome system (CS), as four out of these seven carriers are cytochrome. It is the major source of cells energy, in the respiratory breakdown of simple carbohydrates intermediates like phosphoglyceraldehyde, pyruvic acid, isocitric acid, ketoglutaric acid, succinic acid and malic acid are oxidised. The oxidation in all these brought about by the removal of a pair of hydrogen atoms (2H) from each of them. This final stage of respiration is carried out in ETS, located in the inner membrane of mitochondria (in prokaryotes the ETS is located in mesosomes of plasma membrane). The system consists of series of precisely arranged seven electron carriers (coenzyme) in the inner membrane of the mitochondrion, including the folds or cristae of this membrane. These seven electron-carriers function in a specific sequence and are : Nicotinamide adenine dinucleotide (NAD), Flavin mononucleotide (FMN), Flavin adenine dinucleotide (FAD), Co-enzyme-Q or ubiquinone, Cytochrome-b, Cytochrome-c, Cytochrome-a and Cytochrome-a3
# Five complex theory : According to Hatefi, (1976), Complex I to Complex IV are related to the electron transport.
Complex V related to mainly with ATP synthesis, so it is called ATPase /ATP syntheses complex.
The head piece (F1) of the oxysome consists of 5 hydrophobic subunits ( ), which are responsible for ATPase functioning.
The stalk (F0) contain F5 (oligomycin sensitivity conferring protein) i.e. CSCP and F6. F0 are related to the proton channel and embeded fully in thickness of inner mitochondrial membrane.
Five complex i.e. I, II, III, IV, V, have been isolated from mitochondrial membrane by chemical treatment.
Complex I : NADH/NADPH : CoQ reductase
Complex II : Succinate : CoQ reductase
Complex III : Reduced CoQ (CoQH2) : cytochrome C reductase
Complex IV : Cytochrome C oxidase
Complex V : ATPase
# The first carrier in the chain is a flavoprotein which is reduced by NADH2. Coenzyme passes these electron to the cytochromes arranged in the sequence of b-c-a-a3, finally pass the electron to molecular oxygen. In this transport, the electrons tend to flow from electro-negative to electro-positive system, so there is a decrease in free energy and some energy is released so amount of energy with the electrons goes on decreasing. During electron-transfer, the electron-donor gets oxidised, while electron-acceptor gets reduced so these transfers involve redox-reaction and are catalysed by enzymes, called reductases. Oxidation and reduction are complimentary. This oxidation-reductiion reaction over the ETC is called biological oxidation.
During the electron transfers, the energy released at some steps is so high that ATP is formed by the phosphorylation of ADP in the presence of enzyme ATP synthetase present in the head of F1-particles present on the mitochondrial crista. This process of ATP synthesis during oxidation of coenzyme is called oxidative phosphorylation, so ETS is also called oxidative phosphorylation pathways.
From the cytochrome a3, two electrons are received by oxygen atom which also receives two proton (H+) from the mitochondrial matrix to form water molecule. So the final acceptor electrons is oxygen. So the reaction H2 + O2 ---> H2O (called metabolic water) is made to occur in many steps through ETC, so the most of the energy can be derived into a storage and usable form.
# Two route systems of ETC : The pairs of hydrogen atoms from respiratory intermediates are received either by NAD+ or FAD coenzymes which becomes reduced to NADH2 and FADH2. These reduced coenzyme pass the electrons on to ETC. Thus, regeneration of NAD+ or FAD takes place in ETC. There are two routes ETC :
- (a) Route 1 : NADH2 passes their electrons to Co-Q through FAD . In route 1 FAD is the first electron carrier. 3 ATP molecules are produced during the transfer of electron on following steps :
NAD to FAD
Cyt b to Cyt c and
Cyt a to Cyt a3
- (b) Route 2 : FADH2 passes their electron directly to FAD. 2 ATP molecules are produced during the transfer of electron on following steps.
Cyt b to Cyt c and
Cyt a to Cyt a3
# Development of proton gradient : At each step of ETC, the electron- acceptor has a higher electron –affinity than the electron-donor. The energy from electron-transport is used to move the proton (H+) from the mitochondrial matrix to inter-membranous or outer chamber. Three pairs of protons are pushed to outer chamber during the movement of electrons along route I while two pairs of protons are moved to outer chamber during the movement of electrons along route–II.
This generates a pH-gradient across the inner mitochondrial membrane with protons (H+) concentration higher in the outer chamber than in the mitochondrial matrix. This difference in H+ concentration across the inner mitochondrial membrane is called proton-gradient
( pH). Due to proton gradient, an electrical potential is developed across the inner mitochondrial membrane as the matrix is now electronegative with respect to the intermembranous (outer) chamber. The proton gradient and membrane electric potential collectively called proton motive force.
# (b) Proton flow : Due to proton-gradient, the protons returns to the matrix while passing through proton channel of F0-F1 ATPase. This proton gradient activates the enzyme ATP synthetase or F0 – F1 ATPase
ATP synthetase controls the formation of ATP from ADP and inorganic phosphate in the presence of energy.