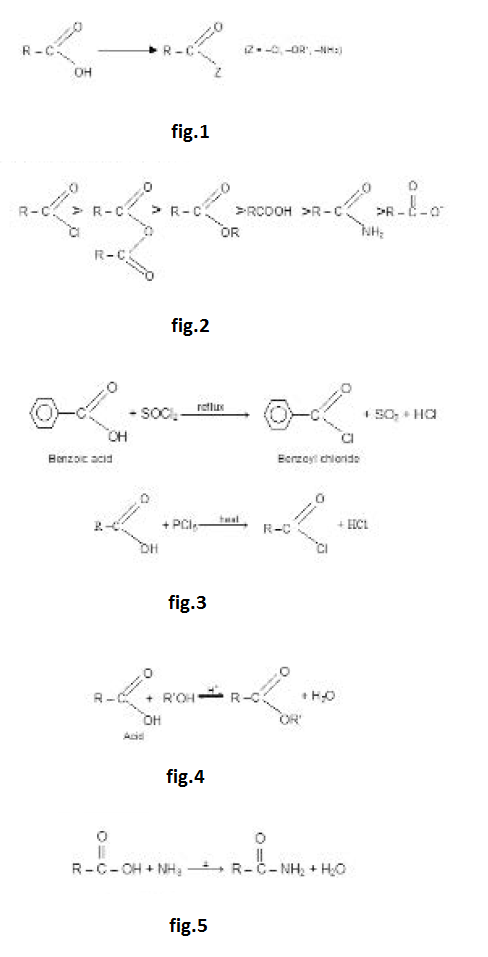
Conversion into Functional Derivatives :
The `-OH` of an acid can be replaced by a `-Cl`, `-OR` or `-NH_2` group to yield an acid chloride, an ester or an amide. These comppunds are called functional derivatives of acid they all contain acyl group.
The functional derivatives are all readily reconverted into the acid by simple hydrolysis. See fig.1.
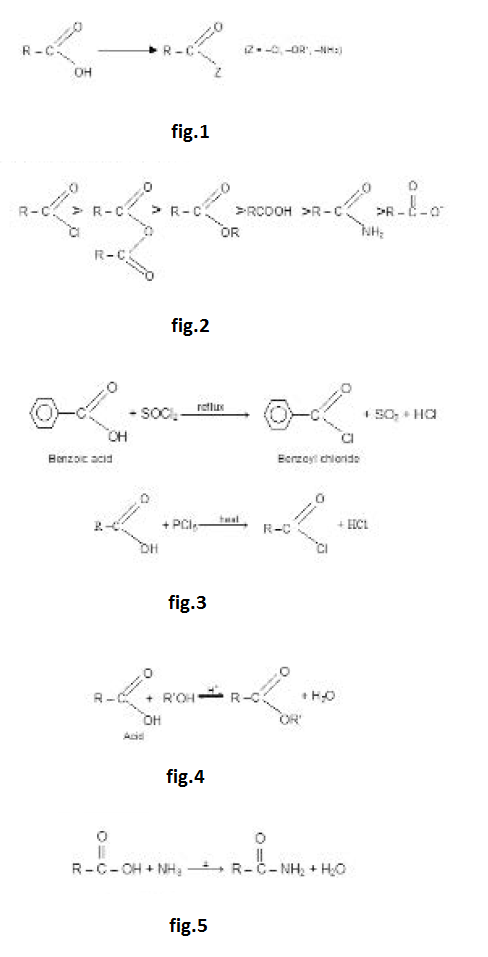
The initial step in both reactions involves nucleophilic addition at the carbonyl carbon atom. With both groups of compound, this initial attack is facilitated by the same factors : the relative steric opens of the carbonyl oxygen atom to accommodate an electron pair of the carbon oxygen double bond. It is after the initial nucleophilic attack has taken place that the two reactions differ. The tetrahedral intermediate formed from an aldehyde or Ketone usually accepts a proton to form a stable addition product. By contrast, the intermediate formed from an acyl compound usually eliminates a leaving group: this elimination leads to regeneration of the carbon oxygen double bond and to a substitution product. The overall process in the case of acyl substitution occurs, therefore, by a nucleophilic addition elimination mechanism. Acyl compounds react as they do so because they all have good leaving groups attached to the carbonyl carbon atom: See fig.2.
The general order of reactivity of acid derivatives can be explained by taking into account the basicity of the leaving groups.
`text(Conversion into Acid Chlorides :)` An acid chloride is prepared by substitution of `-Cl` for the `-OH` in a carboxylic acid. Three reagents are commonly used for this purpose. Thionylchloride, `(SOCl_2)`, phosphorus trichloride, `(PCl_3)` and phosphorus pentachloride, `(PCl_5)`. See fig.3.
Thionyl chloride is particularly convenient, since the product formed besides the acid chloride are gases and thus easily separated from the acid chloride; any excess of the thionyl chloride `[B.P. = 79^oC]` is easily removed by distillation.
`text(Conversion into Esters (Esterification))`
Carboxylic acid on heating with alcohols in presence of dehydrating agent `(H_2SO_4` or dry `HCl` gas) gives esters. The reaction is known as esterification. See fig.4.
This reaction is reversible and the same catalyst, hydrogen ion, that catalyzes the forward reaction i.e. esterification necessarily catalyzes the reverse reaction i.e. hydrolysis.
The equilibrium is particularly unfavorable when phenols `(ArOH)` are used instead of alcohol; yet if water is removed during the reaction, phenolic esters `(RCOOAr)` are obtained in high yield.
The presence of bulky group near the site of reaction, whether in alcohol or in the acid, slows down esterification (as well as its reverse, hydrolysis).
Reactivity `CH_3OH > 1^o > 2^o > 3^o`
In esterification `HCOOH > CH_3COOH > RCH_2COOH > R_2CHCOOH > R_3C COOH`
`text(Conversion into Amides :)` See fig.5.
`text(Conversion into Anhydrides :)` Lower monocarboxylic acid on heating with dehydrating agent (say `P_2O_5`) forms anhydrides.
`2CH_3COOH underset(Delta)overset(P_2O_5)-> (CH_3CO_2)O +H_2O`
`text(Note :)` Anhydride of formic acid is not known, it gives `CO` and `H_2O` on heating with conc. `H_2SO_4`.
`HCOOH overset(H_2SO_4)-> H_2O + CO`
The functional derivatives are all readily reconverted into the acid by simple hydrolysis. See fig.1.
The initial step in both reactions involves nucleophilic addition at the carbonyl carbon atom. With both groups of compound, this initial attack is facilitated by the same factors : the relative steric opens of the carbonyl oxygen atom to accommodate an electron pair of the carbon oxygen double bond. It is after the initial nucleophilic attack has taken place that the two reactions differ. The tetrahedral intermediate formed from an aldehyde or Ketone usually accepts a proton to form a stable addition product. By contrast, the intermediate formed from an acyl compound usually eliminates a leaving group: this elimination leads to regeneration of the carbon oxygen double bond and to a substitution product. The overall process in the case of acyl substitution occurs, therefore, by a nucleophilic addition elimination mechanism. Acyl compounds react as they do so because they all have good leaving groups attached to the carbonyl carbon atom: See fig.2.
The general order of reactivity of acid derivatives can be explained by taking into account the basicity of the leaving groups.
`text(Conversion into Acid Chlorides :)` An acid chloride is prepared by substitution of `-Cl` for the `-OH` in a carboxylic acid. Three reagents are commonly used for this purpose. Thionylchloride, `(SOCl_2)`, phosphorus trichloride, `(PCl_3)` and phosphorus pentachloride, `(PCl_5)`. See fig.3.
Thionyl chloride is particularly convenient, since the product formed besides the acid chloride are gases and thus easily separated from the acid chloride; any excess of the thionyl chloride `[B.P. = 79^oC]` is easily removed by distillation.
`text(Conversion into Esters (Esterification))`
Carboxylic acid on heating with alcohols in presence of dehydrating agent `(H_2SO_4` or dry `HCl` gas) gives esters. The reaction is known as esterification. See fig.4.
This reaction is reversible and the same catalyst, hydrogen ion, that catalyzes the forward reaction i.e. esterification necessarily catalyzes the reverse reaction i.e. hydrolysis.
The equilibrium is particularly unfavorable when phenols `(ArOH)` are used instead of alcohol; yet if water is removed during the reaction, phenolic esters `(RCOOAr)` are obtained in high yield.
The presence of bulky group near the site of reaction, whether in alcohol or in the acid, slows down esterification (as well as its reverse, hydrolysis).
Reactivity `CH_3OH > 1^o > 2^o > 3^o`
In esterification `HCOOH > CH_3COOH > RCH_2COOH > R_2CHCOOH > R_3C COOH`
`text(Conversion into Amides :)` See fig.5.
`text(Conversion into Anhydrides :)` Lower monocarboxylic acid on heating with dehydrating agent (say `P_2O_5`) forms anhydrides.
`2CH_3COOH underset(Delta)overset(P_2O_5)-> (CH_3CO_2)O +H_2O`
`text(Note :)` Anhydride of formic acid is not known, it gives `CO` and `H_2O` on heating with conc. `H_2SO_4`.
`HCOOH overset(H_2SO_4)-> H_2O + CO`