Naming of Carboylic Acid Derivatives :
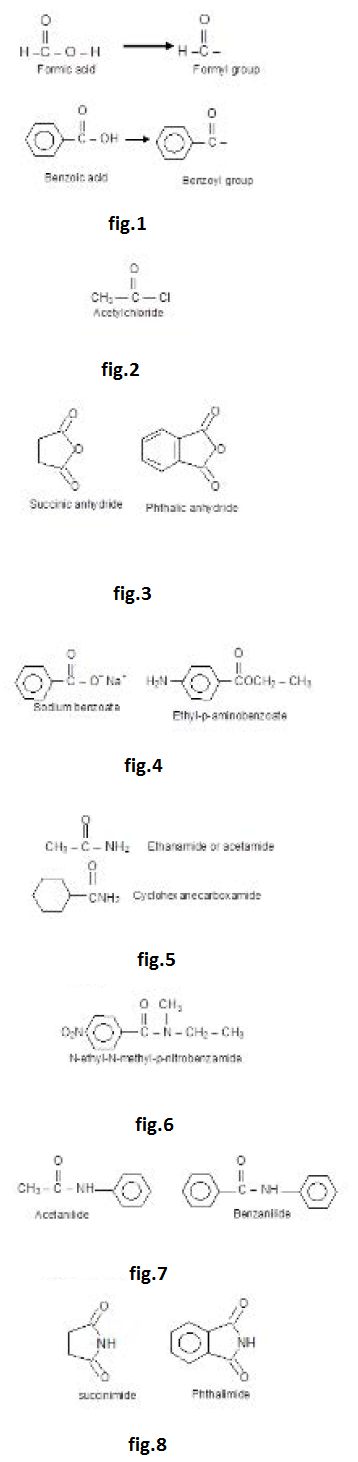
Naming of Acyl Groups; Acid Chlorides and Acid Anhydrides : The group obtained from a carboxylic acid by the removal of the hydroxyl portion is known as an acyl group The name of an acyl group is created by changing the 'ic' at the end of the name of the carboxylic acid to 'yl', examples : See fig.1.
Acid chlorides are named systematically as acyl chlorides. See fig.2.
An acid anhydride is named by substituting anhydride for acid in the name of the acid from which it is derived. See fig.3.
Naming of Salts and Esters : The name of the cation (in the case of a salt) or the name of the organic group attached to the oxygen or the carboxyl group (in the case of an ester) preceeds the name of the acid. The 'ic acid' part of the name of the acid is converted to 'ate'. See fig.4.
Naming of Amides and lmides : The names of amides are formed by replacing -oic acid (or -ic acid for common names) by amide or - carboxylic acid by carboxamide. See fig.5.
If the nitrogen atom of the amide has any alkyl group as substituent, the name of the amide is prefixed by the capital letter `N` to Indicate substitution on nitrogen, followed by the name(s) of alkyl group(s). See fig.6.
If the substituent on the nitrogen atom of an amide is a phenyl group, the ending for the name of the carboxylic acid is changed to anilide. See fig.7.
Some dicarboxylic acids form cyclic amides in which two acyl groups are bonded to the nitrogen atom. The suffix imide is given to such compounds. See fig.8.
Acid chlorides are named systematically as acyl chlorides. See fig.2.
An acid anhydride is named by substituting anhydride for acid in the name of the acid from which it is derived. See fig.3.
Naming of Salts and Esters : The name of the cation (in the case of a salt) or the name of the organic group attached to the oxygen or the carboxyl group (in the case of an ester) preceeds the name of the acid. The 'ic acid' part of the name of the acid is converted to 'ate'. See fig.4.
Naming of Amides and lmides : The names of amides are formed by replacing -oic acid (or -ic acid for common names) by amide or - carboxylic acid by carboxamide. See fig.5.
If the nitrogen atom of the amide has any alkyl group as substituent, the name of the amide is prefixed by the capital letter `N` to Indicate substitution on nitrogen, followed by the name(s) of alkyl group(s). See fig.6.
If the substituent on the nitrogen atom of an amide is a phenyl group, the ending for the name of the carboxylic acid is changed to anilide. See fig.7.
Some dicarboxylic acids form cyclic amides in which two acyl groups are bonded to the nitrogen atom. The suffix imide is given to such compounds. See fig.8.