Physical Properties :
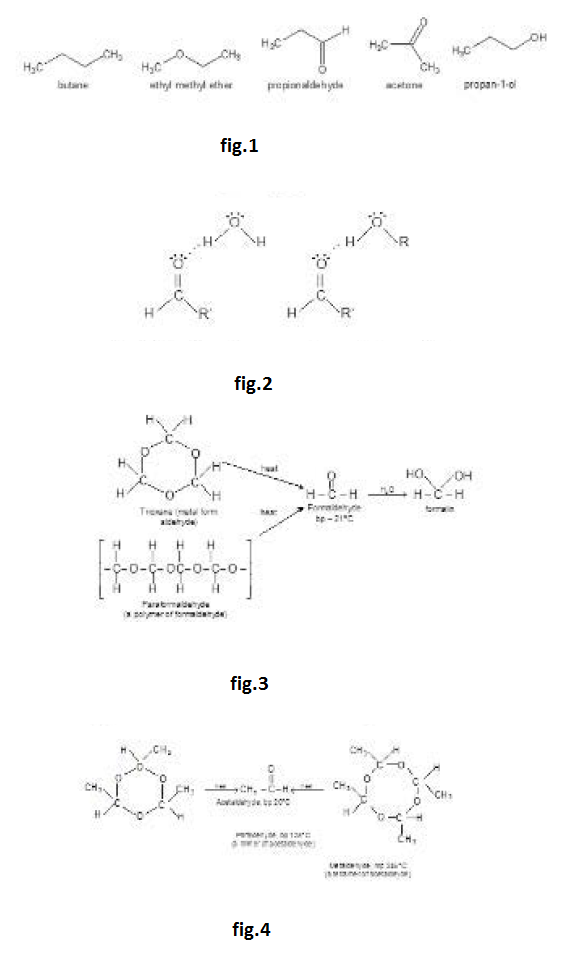
Polarization of the carbonyl group creates dipole-dipole interactions between the molecules of ketones and aldehydes, resulting in higher boiling points than for hydrocarbons and ethers of similar molecular weights. Ketones and aldehydes have no `O - H` or `N - H` bonds, however, so they cannot form hydrogen bonds with each other. The following compounds of molecular weight `58` or `60` are ranked in order of increasing boiling point. The ketones and the aldehyde are more polar and higher-boiling than the ether and the alkane, but lower boiling than the hydrogen-bonded alcohol. See fig.1.
The melting points, boiling points, and water solubilities of some representative ketones and aldehydes.
Although pure ketones and aldehydes cannot engage in hydrogen bonding with each other, they have lone pairs of electrons and can act as hydrogen bond acceptors with other compounds have `O - H` or `N - H` bonds. For example, the `-OH` of water or an alcohol can form a hydrogen bond with the unshared electrons on a carbonyl oxygen atom. See fig.2.
Because of this hydrogen bonding, ketones and aldehydes are good solvents for polar hydroxylic substances such as alcohols. They can also remarkably soluble in water. That acetaldehyde and acetone are miscible (soluble in all proportions) with water, and other ketones and aldehydes with up to four carbon atoms are appreciably soluble in water. These solubility properties are similar to those of ethers and alcohols, which also engage in hydrogen bonding with water.
Formaldehyde and acetaldehyde are the most common aldehydes. Formaldehyde is a gas at room temperature, so it is often stored and used as a `40` percent aqueous solution called formalin. When dry formaldehyde is needed, it can be generated by heating one of the solid derivatives of formaldehyde, usually trioxane units. Paraformaldehyde is a linear polymer, containing many formaldehyde units. These solid derivatives form spotaneously when a small amount of acid catalyst is added to pure formaldehyde. See fig.3.
Acetaldehyde boils near room temperature, and it can be handled as a liquid. Acetaldehyde is also used as a trimer (paraldehyde) and a tetramer (metaldehyde), formed from acetaldehyde under acid catalysis. Heating either of these compounds provides dry acetaldehyde. Paraldehyde is used in medicines as a sedative, and metaldehyde is used as a bailt and poison for snails and slugs. See fig.4.
The melting points, boiling points, and water solubilities of some representative ketones and aldehydes.
Although pure ketones and aldehydes cannot engage in hydrogen bonding with each other, they have lone pairs of electrons and can act as hydrogen bond acceptors with other compounds have `O - H` or `N - H` bonds. For example, the `-OH` of water or an alcohol can form a hydrogen bond with the unshared electrons on a carbonyl oxygen atom. See fig.2.
Because of this hydrogen bonding, ketones and aldehydes are good solvents for polar hydroxylic substances such as alcohols. They can also remarkably soluble in water. That acetaldehyde and acetone are miscible (soluble in all proportions) with water, and other ketones and aldehydes with up to four carbon atoms are appreciably soluble in water. These solubility properties are similar to those of ethers and alcohols, which also engage in hydrogen bonding with water.
Formaldehyde and acetaldehyde are the most common aldehydes. Formaldehyde is a gas at room temperature, so it is often stored and used as a `40` percent aqueous solution called formalin. When dry formaldehyde is needed, it can be generated by heating one of the solid derivatives of formaldehyde, usually trioxane units. Paraformaldehyde is a linear polymer, containing many formaldehyde units. These solid derivatives form spotaneously when a small amount of acid catalyst is added to pure formaldehyde. See fig.3.
Acetaldehyde boils near room temperature, and it can be handled as a liquid. Acetaldehyde is also used as a trimer (paraldehyde) and a tetramer (metaldehyde), formed from acetaldehyde under acid catalysis. Heating either of these compounds provides dry acetaldehyde. Paraldehyde is used in medicines as a sedative, and metaldehyde is used as a bailt and poison for snails and slugs. See fig.4.