Aldehydes and ketones have positively charged carbon which is planar since it is `sp^2` hybridized. So, this positive charged carbon can be easily attacked by nucleophile. So carbonyl compounds can undergo either nucleophilic substitution or nucleophilic addition reaction. But a carbonyl compound does not undergo nucleophilic substitution reactions because it doesn't have good leaving group. See fig.1.
Now, it's clear to you that these compounds undergo Nu- addition reactions. Let us see these reactions in detail. One more thing to add at this point. Ketones undergoes these reactions very slowly because of the lesser positive charge on the carbonyl carbon, since it is attached to two `+I` effect groups. The approach of `Nu^(-)` will be slow. See fig.2.
Now let me introduce the nucleophilic addition reactions of carbonyl compounds to you.
`text(Addition of Grignard reagents :)` Aldehydes and ketones add on a molecule of a Grignard reagent to give an adduct, which on decomposition by aqueous acid gives an alcohol. See fig.3.
The product is a primary alcohol, if carbonyl compound is formaldehyde. An aldehyde (other than formaldehyde) gives secondary alcohol and a ketone leads to the formation of tertiary alcohol.
`text(Addition of hydrogen cyanide :)`
Aldehydes and ketones add on hydrogen cyanide to form addition product, cyanohydrin. See fig.4.
`HCN` is not the nucleophile in this reaction. lnfact the nucleophile is `CN^(-)`, which is more nucleophilic than `HCN`. The rate law for the reaction is : See fig.5.
The addition of `HCN` to carbonyl compounds is base catalysed, which can lead to the generation of more nucleophilic `CN^(-)`.
`text(Mechanism :)` See fig.6.
The addition of `CN^(-)` is reversible and the equilibrium lies in the direction of carbonyl compound. The presence of a proton donor shifts the equilibrium in the forward direction by converting into cyanohydrin. The RDS of the reaction is attack by `CN^(-)` while proton transfer is a rapid step. The rate of reaction of `HCN` with aldehydes, simple aliphatic and cyclic ketones are fast but slow with `ArCOR` and reaction does not take place at all with `ArCOAr`.
The `CN^(-)` ion may attack from front side or back side as carbonyl group is planar to give enantiomeric pair (provided the groups attached to carbonyl group are different). See fig.7.
The cyanohydrins can be readily hydrolysed to give `alpha`-hydroxy acids. See fig.8.
`text(Addition of sodium bisulphite :)` Aldehydes and ketones add on sodium hydrogen sulphite to form bisulphite compounds. See fig.9.
This bisulphite formation is confined to aldehydes, methyl ketones and some cyclic ketones. Those carbonyl compounds, which form bisulphite adduct can be separated from mixtures as they are crystalline solids, insoluble in `NaHSO_3` solution and/or purified by isolation, purification and subsequent decomposition of these adducts in dilute acids.
`text(Mechanism :)`
The effective nucleophile of the reaction is `SO_3^(2-)` ion rather than `HSO_3^-` and formation of `SO_3^(2-)` is achieved in slightly basic solution. `NaHSO_3 -> Na^(+) + HCO_3^(-)` ` HSO_3^(-) ⇋ H^(+) SO_3^(2-)`
See fig.10.
For example, See fig.11.
Hindered ketones like di-isopropyl ketone, di-t-butyl ketone does not undergo any reaction with `NaHSO_3`.
`text(Addition of ammonia derivatives :)` Aldehydes and ketones combine with a variety of ammonia derivatives of the type `NH_2 -Z`
(where `Z = -OH, - NH_2, - NHC_6H_5, - NHCONH_2` etc). The general reaction is shown as : See fig.12.
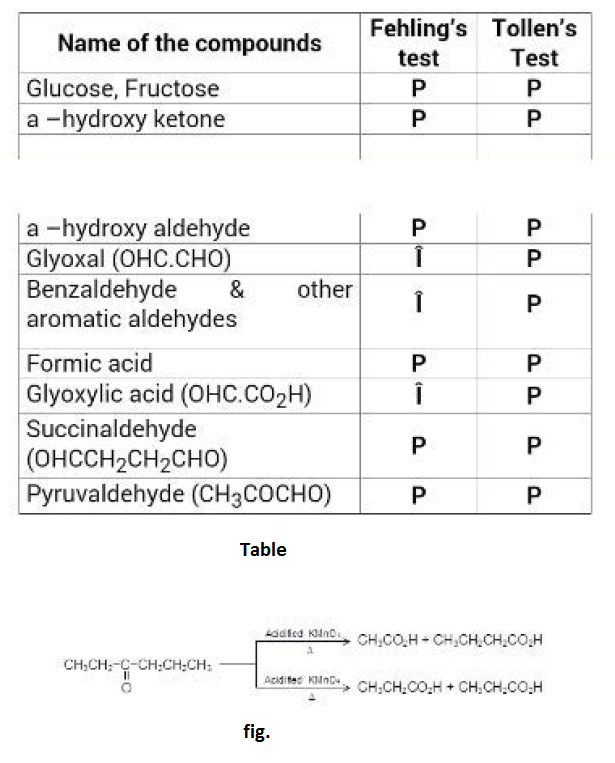
The various ammonia derivatives and their products are indicated in the following table. See Table.
Oximes are obtained with hydroxylamine.
See fig.13.
With hydrazine, hydrazones and azines are formed.
See fig.14.
Phenyl hydrazine forms phenyl hydrazones.
See fig.15.
Semicarbazide forms semicarbazones.
See fig.16.
Brady's regent with carbonyl compounds gives 2, 4 - dinitro phenyl hydrazone, which is obtained as a yellow crystalline solid. It is thus used for the identification of aldehydes and ketones.
The rate of such reaction is maximum at some particular `pH`. These reactions are catalysed by the presence of slightly acidic conditions. In slightly acidic conditions, dehydration step is the RDS, whose rate is increased by the protonation of `OH`, leading to overall increase in rate of the reaction. But, when the acidity increases, the rate of addition step decreases because concentration of `NH_2- Z` reduces due to its conversion to conjugate acid, `NH_3^+ -Z` (which can not function as a nucleophile because of absence of lone pair). Thus, at low `pH` or more acidity, the addition step becomes the RDS.
`text(Addition of Ammonia :)`
Aldehydes react with ammonia to form aldehyde ammonia
See fig.17.
The aldehyde amounts as unstable and lose water immediately to form aldimine. The dehydration product is not usually obtained because, in most cases, it immediately polymerises to form cyclic trimers.
See fig.18.
When treated with ammonia, formaldehyde does not form an aldehyde-ammonia, but gives instead hexamethylenetetramine, used in medicine as an urinary antiseptic under the name Urotropine.
`6HCHO +4NH_3 -> (CH_2)_6N_4 + 5H_2O(80 %)`
See fig.19.
Ketones also give ketone-ammonia but these cannot be isolated. Acetone reacts slowly with ammonia to form acetone ammonia and then a complex compound.
See fig.20.
Acetone upon treatment with ammonia at higher temperature give acetoneammonia. See fig.21.
Aldimines, Schiff's bases or azomethines are formed when aldehydes react with aliphatic primary amines, which is removed by slow distillation.
`text(Nucleophilic Addition of Water: Hydrogen of Ketones and aldehydes)`
In an aqueous solution, a ketone or an aldehyde is in equilibrium with its hydrate, a geminal diol. With most ketones the equilibrium favors the unhydrated keto form of the carbonyl. See fig.22.
`text(Example)` : See fig.23.
Addition occurs through the nucleophilic addition mechanism with water (in acid) or hydroxide ion (in base) serving as the nucleophile. See fig.24.
Aldehydes are more likely than ketones to form stable hydrates. The electrophilic carbonyl group of a ketone is stabilized by its two electron-donating alkyl groups, but an aldehyde carbonyl has only one stabilizing alkyl group; its partial positive charge is not as well stabilized. Aldehydes are thus more electrophilic and less stable than ketones. Formaldehyde, with no electron-donating groups, is even less stable than other aldehydes. See fig.25.
These stability effects are apparent in the equilibrium constants for hydrogen of ketones and aldehydes. Ketones have values of `K_(eq)` of about `10^(-4)` to `10^(-2)`. For most aldehydes, the equilibrium constant for hydration is close to 1. Formaldehyde, with no alkyl groups bonded to the carbonyl carbon, has a hydration equilibrium constant of about `2000`. Strongly electron withdrawing substituents on the alkyl group of a ketone or aldehyde also destabilize the carbonyl group and favour of hydrate. Chloral (trichloroacetaldehyde) has an electron-withdrawing trichloromethyl group that favours the hydrate. Chloral forms a stable, crystalline hydrate that became famous in the movies as "knockout drops" or a "Mickey Finn". See fig.26.
`text(Reaction with)` `PCl_5` : Phosphorous pentachloride reacts with simple carbonyl compounds to give 1, 1 - dichlorides (gem dichloride). See fig.27.
The possible mechanism of the reaction is : See fig.28.
`text(Reaction with)` `SeO_2` : Aldehydes and ketones with a methyl or methylene group adjacent to the carbonyl group are oxidised by selenium dioxide in acetic acid at room temperature to dicarbonyl compounds. For example, acetaldehyde forms glyoxal and acetone forms methyl glyoxal.
`CH_3CHO + SeO_2 oversettext(acetic acid)-> OHC- CHO + Se + H_2O`
`CH_3COCH_3 +SeO_2 oversettext(acetic acid)-> CH_3COCHO +Se +H_2O`
The actual reagent of the reaction is selenous acid `(H_2SeO_3)` and the probable mechanism is : See fig.29.
`text(Reaction with Chloroform :)`
Ketones condense with chloroform in the presence of potassium hydroxide to give chloretone, which is used as a hypnotic drug.
`CH_3COC H_3 +CHCl_3 overset(KOH)-> undersettext(Chloretone)[(CH_3)_2C(OH)C Cl_3]`
The reaction proceeds as See fig.30.
`text(Addition of Alcohols :)` Carbonyl compounds add on one mole of alcohol to give hemi- acetals and hemi - ketals while addition of two moles of alcohols give acetals and ketals. See fig.31.
Generally, hemi - acetals and hemi - ketals are not isolated while isolable products are acetals and ketals.
Conversion of hemi - acetal to acetal is a specific acid catalysis reaction. Hemi - acetal is first protonated, which then loses `H_2O` molecule to give carbocation. Formation of this carbocation is the rate limiting step. Carbocation is then attacked by neucleophile to give final product, acetal. See fig.32.
This reaction is not so favourable with ketones under these conditions (with simple alcohols) but reaction can be favoured if 1, 2 or 1, 3 - diols are used. With diols, cyclic ketals are formed. The reaction with simple alcohols was not favourable because entropy decreases (`Delta S = - ve`) as 3 molecules give 2 molecules on reaction while with diols, entropy change is zero, so reaction becomes favourable. See fig.33.
Ketals are formed only by unhindered ketones.
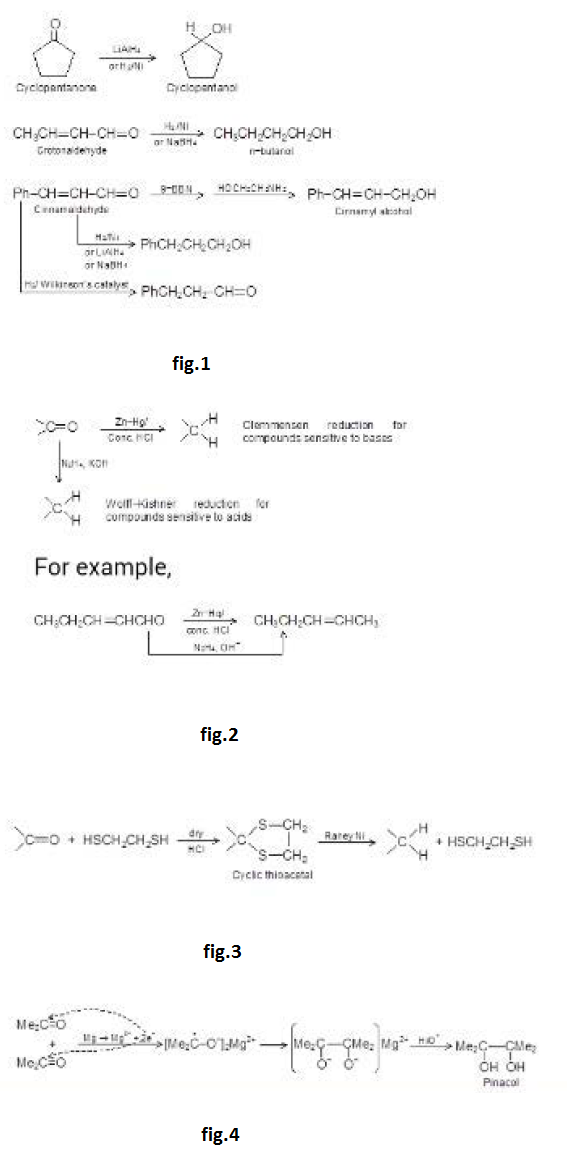
Acetals or ketals are stable in neutral or basic conditions but in acidic medium, they undergo acid catalysed cleavage similar to that of ethers to regenerate carbonyl compounds. Thus, this reaction is used to protect carbonyl groups. For example, we want to convert 2- carbethoxy cyclopentanone to 2-hydroxy methyl cyclopentanone. This can be achieved by protecting keto group and reducing - `CO_2Et` group to `- CH_2OH` by `LiAlH_4.` See fig.34.
For example,
`CH_3 - CH=O + 2C_2H_5OH overset(text(dry)HCl text(gas))-> CH_3CH(OC_2H_5)_2 + H_2O`
See fig.35.
Aldehydes and ketones have positively charged carbon which is planar since it is `sp^2` hybridized. So, this positive charged carbon can be easily attacked by nucleophile. So carbonyl compounds can undergo either nucleophilic substitution or nucleophilic addition reaction. But a carbonyl compound does not undergo nucleophilic substitution reactions because it doesn't have good leaving group. See fig.1.
Now, it's clear to you that these compounds undergo Nu- addition reactions. Let us see these reactions in detail. One more thing to add at this point. Ketones undergoes these reactions very slowly because of the lesser positive charge on the carbonyl carbon, since it is attached to two `+I` effect groups. The approach of `Nu^(-)` will be slow. See fig.2.
Now let me introduce the nucleophilic addition reactions of carbonyl compounds to you.
`text(Addition of Grignard reagents :)` Aldehydes and ketones add on a molecule of a Grignard reagent to give an adduct, which on decomposition by aqueous acid gives an alcohol. See fig.3.
The product is a primary alcohol, if carbonyl compound is formaldehyde. An aldehyde (other than formaldehyde) gives secondary alcohol and a ketone leads to the formation of tertiary alcohol.
`text(Addition of hydrogen cyanide :)`
Aldehydes and ketones add on hydrogen cyanide to form addition product, cyanohydrin. See fig.4.
`HCN` is not the nucleophile in this reaction. lnfact the nucleophile is `CN^(-)`, which is more nucleophilic than `HCN`. The rate law for the reaction is : See fig.5.
The addition of `HCN` to carbonyl compounds is base catalysed, which can lead to the generation of more nucleophilic `CN^(-)`.
`text(Mechanism :)` See fig.6.
The addition of `CN^(-)` is reversible and the equilibrium lies in the direction of carbonyl compound. The presence of a proton donor shifts the equilibrium in the forward direction by converting into cyanohydrin. The RDS of the reaction is attack by `CN^(-)` while proton transfer is a rapid step. The rate of reaction of `HCN` with aldehydes, simple aliphatic and cyclic ketones are fast but slow with `ArCOR` and reaction does not take place at all with `ArCOAr`.
The `CN^(-)` ion may attack from front side or back side as carbonyl group is planar to give enantiomeric pair (provided the groups attached to carbonyl group are different). See fig.7.
The cyanohydrins can be readily hydrolysed to give `alpha`-hydroxy acids. See fig.8.
`text(Addition of sodium bisulphite :)` Aldehydes and ketones add on sodium hydrogen sulphite to form bisulphite compounds. See fig.9.
This bisulphite formation is confined to aldehydes, methyl ketones and some cyclic ketones. Those carbonyl compounds, which form bisulphite adduct can be separated from mixtures as they are crystalline solids, insoluble in `NaHSO_3` solution and/or purified by isolation, purification and subsequent decomposition of these adducts in dilute acids.
`text(Mechanism :)`
The effective nucleophile of the reaction is `SO_3^(2-)` ion rather than `HSO_3^-` and formation of `SO_3^(2-)` is achieved in slightly basic solution. `NaHSO_3 -> Na^(+) + HCO_3^(-)` ` HSO_3^(-) ⇋ H^(+) SO_3^(2-)`
See fig.10.
For example, See fig.11.
Hindered ketones like di-isopropyl ketone, di-t-butyl ketone does not undergo any reaction with `NaHSO_3`.
`text(Addition of ammonia derivatives :)` Aldehydes and ketones combine with a variety of ammonia derivatives of the type `NH_2 -Z`
(where `Z = -OH, - NH_2, - NHC_6H_5, - NHCONH_2` etc). The general reaction is shown as : See fig.12.
The various ammonia derivatives and their products are indicated in the following table. See Table.
Oximes are obtained with hydroxylamine.
See fig.13.
With hydrazine, hydrazones and azines are formed.
See fig.14.
Phenyl hydrazine forms phenyl hydrazones.
See fig.15.
Semicarbazide forms semicarbazones.
See fig.16.
Brady's regent with carbonyl compounds gives 2, 4 - dinitro phenyl hydrazone, which is obtained as a yellow crystalline solid. It is thus used for the identification of aldehydes and ketones.
The rate of such reaction is maximum at some particular `pH`. These reactions are catalysed by the presence of slightly acidic conditions. In slightly acidic conditions, dehydration step is the RDS, whose rate is increased by the protonation of `OH`, leading to overall increase in rate of the reaction. But, when the acidity increases, the rate of addition step decreases because concentration of `NH_2- Z` reduces due to its conversion to conjugate acid, `NH_3^+ -Z` (which can not function as a nucleophile because of absence of lone pair). Thus, at low `pH` or more acidity, the addition step becomes the RDS.
`text(Addition of Ammonia :)`
Aldehydes react with ammonia to form aldehyde ammonia
See fig.17.
The aldehyde amounts as unstable and lose water immediately to form aldimine. The dehydration product is not usually obtained because, in most cases, it immediately polymerises to form cyclic trimers.
See fig.18.
When treated with ammonia, formaldehyde does not form an aldehyde-ammonia, but gives instead hexamethylenetetramine, used in medicine as an urinary antiseptic under the name Urotropine.
`6HCHO +4NH_3 -> (CH_2)_6N_4 + 5H_2O(80 %)`
See fig.19.
Ketones also give ketone-ammonia but these cannot be isolated. Acetone reacts slowly with ammonia to form acetone ammonia and then a complex compound.
See fig.20.
Acetone upon treatment with ammonia at higher temperature give acetoneammonia. See fig.21.
Aldimines, Schiff's bases or azomethines are formed when aldehydes react with aliphatic primary amines, which is removed by slow distillation.
`text(Nucleophilic Addition of Water: Hydrogen of Ketones and aldehydes)`
In an aqueous solution, a ketone or an aldehyde is in equilibrium with its hydrate, a geminal diol. With most ketones the equilibrium favors the unhydrated keto form of the carbonyl. See fig.22.
`text(Example)` : See fig.23.
Addition occurs through the nucleophilic addition mechanism with water (in acid) or hydroxide ion (in base) serving as the nucleophile. See fig.24.
Aldehydes are more likely than ketones to form stable hydrates. The electrophilic carbonyl group of a ketone is stabilized by its two electron-donating alkyl groups, but an aldehyde carbonyl has only one stabilizing alkyl group; its partial positive charge is not as well stabilized. Aldehydes are thus more electrophilic and less stable than ketones. Formaldehyde, with no electron-donating groups, is even less stable than other aldehydes. See fig.25.
These stability effects are apparent in the equilibrium constants for hydrogen of ketones and aldehydes. Ketones have values of `K_(eq)` of about `10^(-4)` to `10^(-2)`. For most aldehydes, the equilibrium constant for hydration is close to 1. Formaldehyde, with no alkyl groups bonded to the carbonyl carbon, has a hydration equilibrium constant of about `2000`. Strongly electron withdrawing substituents on the alkyl group of a ketone or aldehyde also destabilize the carbonyl group and favour of hydrate. Chloral (trichloroacetaldehyde) has an electron-withdrawing trichloromethyl group that favours the hydrate. Chloral forms a stable, crystalline hydrate that became famous in the movies as "knockout drops" or a "Mickey Finn". See fig.26.
`text(Reaction with)` `PCl_5` : Phosphorous pentachloride reacts with simple carbonyl compounds to give 1, 1 - dichlorides (gem dichloride). See fig.27.
The possible mechanism of the reaction is : See fig.28.
`text(Reaction with)` `SeO_2` : Aldehydes and ketones with a methyl or methylene group adjacent to the carbonyl group are oxidised by selenium dioxide in acetic acid at room temperature to dicarbonyl compounds. For example, acetaldehyde forms glyoxal and acetone forms methyl glyoxal.
`CH_3CHO + SeO_2 oversettext(acetic acid)-> OHC- CHO + Se + H_2O`
`CH_3COCH_3 +SeO_2 oversettext(acetic acid)-> CH_3COCHO +Se +H_2O`
The actual reagent of the reaction is selenous acid `(H_2SeO_3)` and the probable mechanism is : See fig.29.
`text(Reaction with Chloroform :)`
Ketones condense with chloroform in the presence of potassium hydroxide to give chloretone, which is used as a hypnotic drug.
`CH_3COC H_3 +CHCl_3 overset(KOH)-> undersettext(Chloretone)[(CH_3)_2C(OH)C Cl_3]`
The reaction proceeds as See fig.30.
`text(Addition of Alcohols :)` Carbonyl compounds add on one mole of alcohol to give hemi- acetals and hemi - ketals while addition of two moles of alcohols give acetals and ketals. See fig.31.
Generally, hemi - acetals and hemi - ketals are not isolated while isolable products are acetals and ketals.
Conversion of hemi - acetal to acetal is a specific acid catalysis reaction. Hemi - acetal is first protonated, which then loses `H_2O` molecule to give carbocation. Formation of this carbocation is the rate limiting step. Carbocation is then attacked by neucleophile to give final product, acetal. See fig.32.
This reaction is not so favourable with ketones under these conditions (with simple alcohols) but reaction can be favoured if 1, 2 or 1, 3 - diols are used. With diols, cyclic ketals are formed. The reaction with simple alcohols was not favourable because entropy decreases (`Delta S = - ve`) as 3 molecules give 2 molecules on reaction while with diols, entropy change is zero, so reaction becomes favourable. See fig.33.
Ketals are formed only by unhindered ketones.
Acetals or ketals are stable in neutral or basic conditions but in acidic medium, they undergo acid catalysed cleavage similar to that of ethers to regenerate carbonyl compounds. Thus, this reaction is used to protect carbonyl groups. For example, we want to convert 2- carbethoxy cyclopentanone to 2-hydroxy methyl cyclopentanone. This can be achieved by protecting keto group and reducing - `CO_2Et` group to `- CH_2OH` by `LiAlH_4.` See fig.34.
For example,
`CH_3 - CH=O + 2C_2H_5OH overset(text(dry)HCl text(gas))-> CH_3CH(OC_2H_5)_2 + H_2O`
See fig.35.