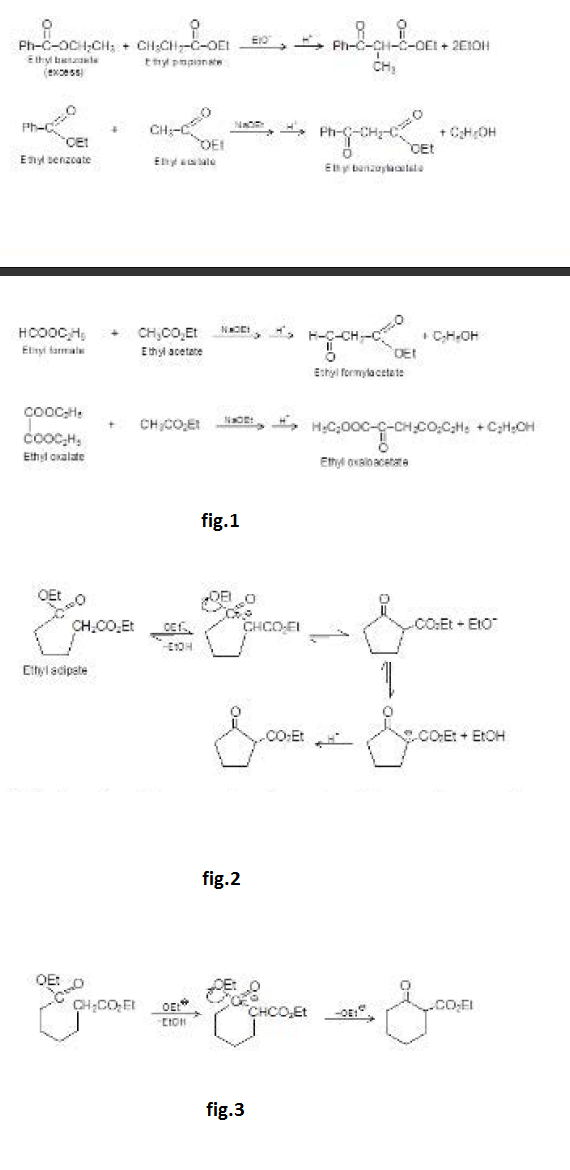
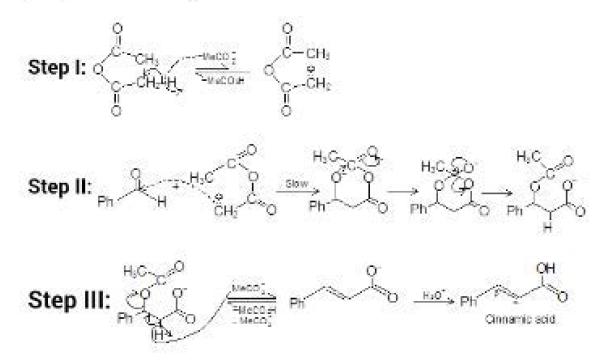
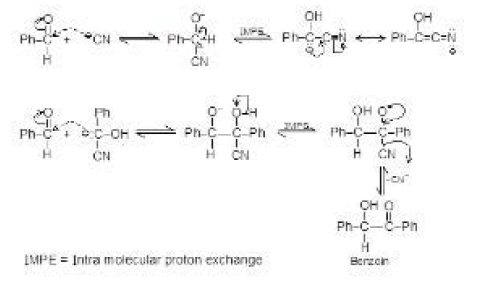
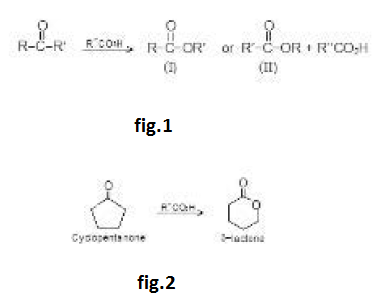
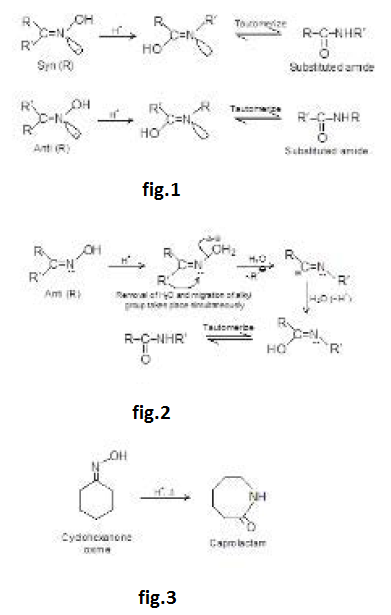
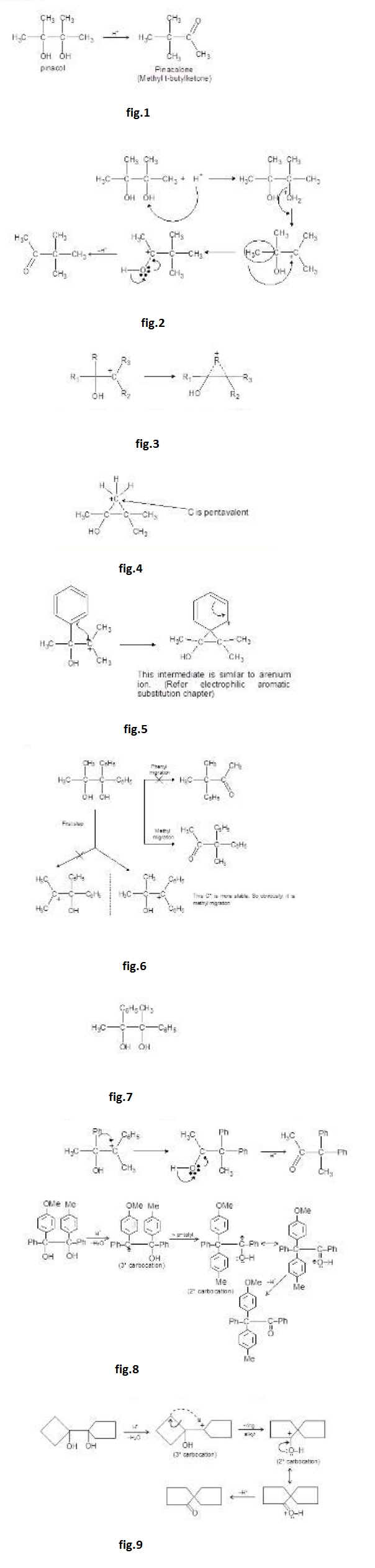
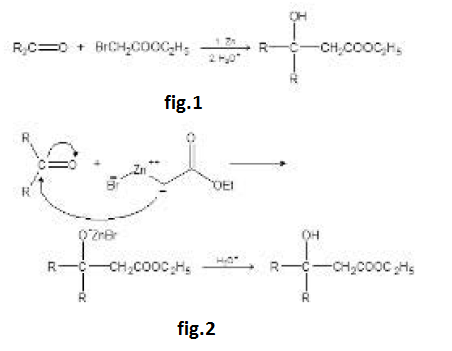
Aldol Condensation :
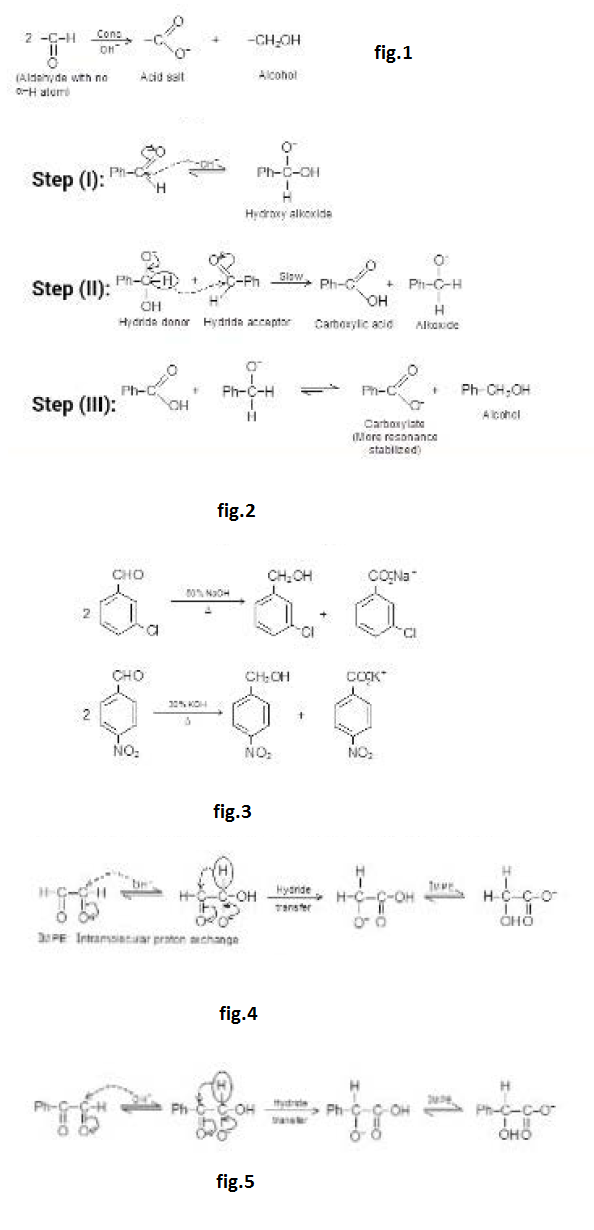
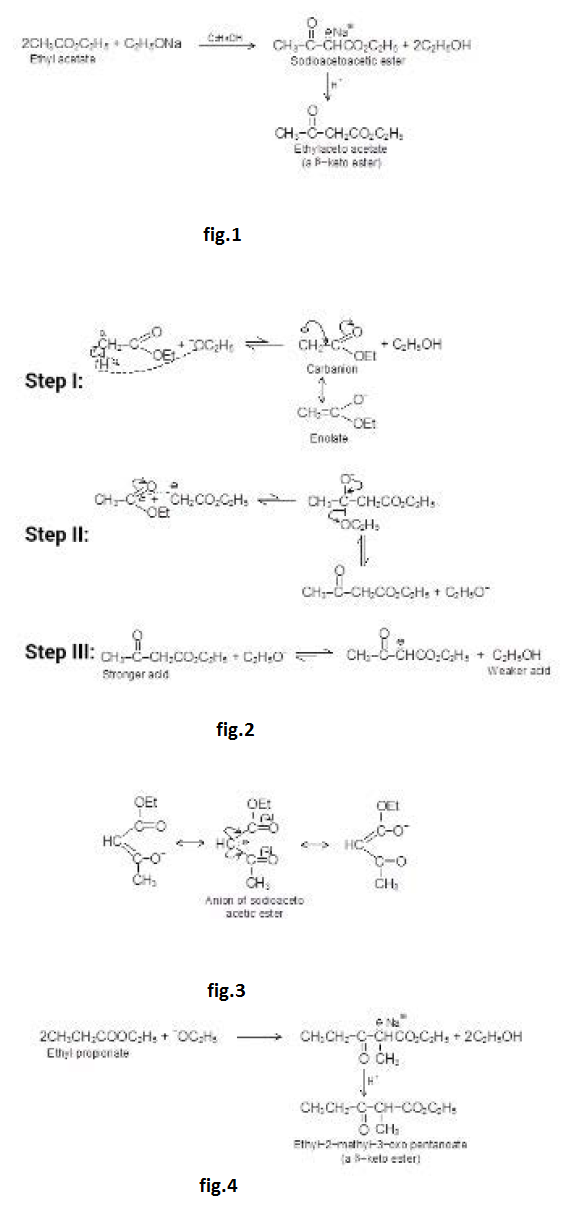
Under the influence of dilute base, two molecules of an aldehyde or a ketone may combine to form a `beta`-hydroxy aldehyde or `beta`- hydroxy ketone. This reaction is called the aldol reaction because product of reaction of 2 moles of aldehyde is called aldol ("aid" for aldehyde and "ol" for alcohol). In every case, the product results from addition of one molecule of aldehyde (or ketone) to a second molecule in such a manner that the `alpha`- carbon of the first becomes attached to the carbonyl carbon of the second. Because the addition reaction is reversible, good yields of the addition product are obtained only if it is removed from the solution as it is formed.
The aldol reaction in more favourable for aldehydes than for ketones because of more acidic `alpha`-hydrogen atoms and more electrophilic carbon.
Heating the aldol product in either acid or base leads to dehydration because the double bond generated is in conjugation with the carbonyl group (making it more stable). If the product of an aldol addition is dehydrated, the overall reaction is called an aldol condensation.
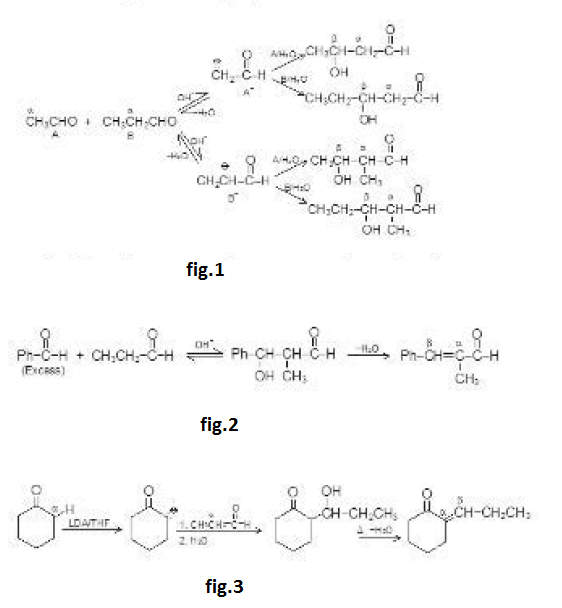
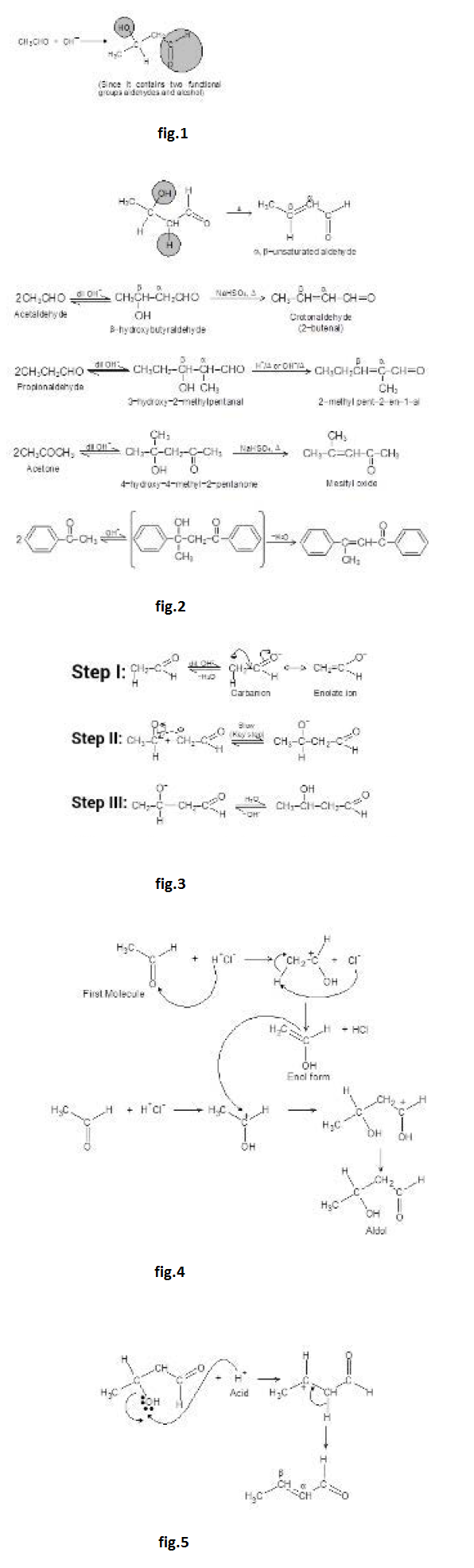
The dehydration product is `alpha`, `beta`- unsaturated carbonyl compound. When the aldol product contains an aryl (or phenyl) group at the `beta`- position, dehydration occurs under the conditions in which the aldol addition is carried out, without additional heating. This is because the double bond formed is conjugated not only with the carbonyl group but also with the aryl group. This makes the product a very stable compound and is therefore easy to form. For example, See fig.1.
Aldol easily undergoes dehydration. See fig.2.
The carbonyl group plays two important roles in the aldol condensation. First, it makes `alpha`-hydrogens acidic enough for carbanion formation to take place and secondly, it provides the unsaturated linkage at which nucleophilic addition takes place. See fig.3.
This reaction can also be catalysed by acid. Let us see this mechanism and the product formed. See fig.4.
But in acid catalysed reaction, the aldol formed is protonated by acid and dehydrated to `alpha`, `beta`-unsaturated carbonyl compounds. See fig.5.
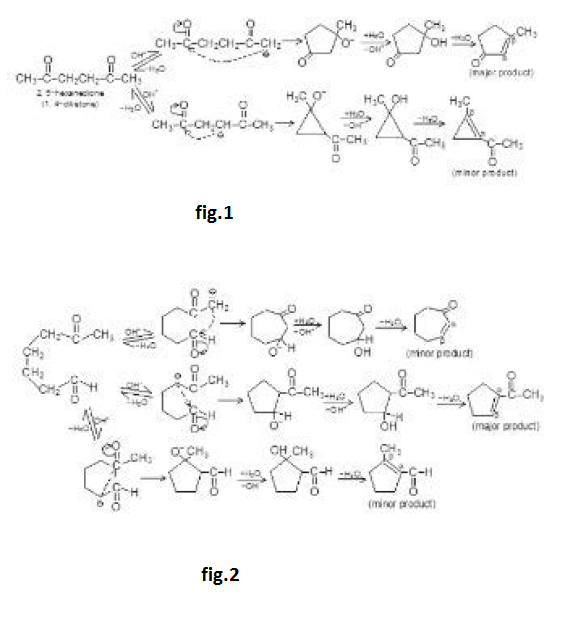
So, in base catalysed reaction, aldol is formed which on further heating it gives `alpha`, `beta`-unsaturated carbonyl compound is obtained (aldol is just an intermediate).
The aldol reaction in more favourable for aldehydes than for ketones because of more acidic `alpha`-hydrogen atoms and more electrophilic carbon.
Heating the aldol product in either acid or base leads to dehydration because the double bond generated is in conjugation with the carbonyl group (making it more stable). If the product of an aldol addition is dehydrated, the overall reaction is called an aldol condensation.
The dehydration product is `alpha`, `beta`- unsaturated carbonyl compound. When the aldol product contains an aryl (or phenyl) group at the `beta`- position, dehydration occurs under the conditions in which the aldol addition is carried out, without additional heating. This is because the double bond formed is conjugated not only with the carbonyl group but also with the aryl group. This makes the product a very stable compound and is therefore easy to form. For example, See fig.1.
Aldol easily undergoes dehydration. See fig.2.
The carbonyl group plays two important roles in the aldol condensation. First, it makes `alpha`-hydrogens acidic enough for carbanion formation to take place and secondly, it provides the unsaturated linkage at which nucleophilic addition takes place. See fig.3.
This reaction can also be catalysed by acid. Let us see this mechanism and the product formed. See fig.4.
But in acid catalysed reaction, the aldol formed is protonated by acid and dehydrated to `alpha`, `beta`-unsaturated carbonyl compounds. See fig.5.
So, in base catalysed reaction, aldol is formed which on further heating it gives `alpha`, `beta`-unsaturated carbonyl compound is obtained (aldol is just an intermediate).