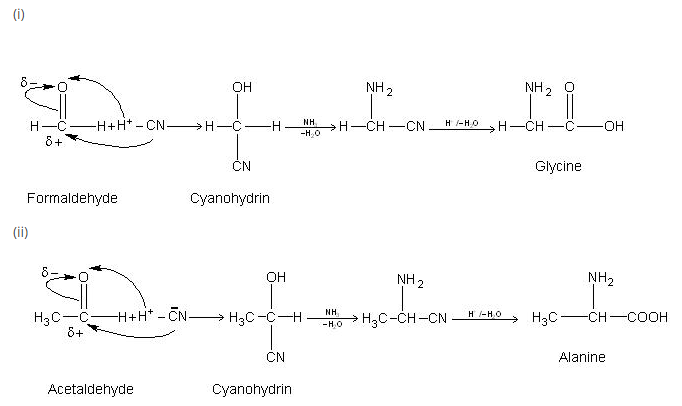
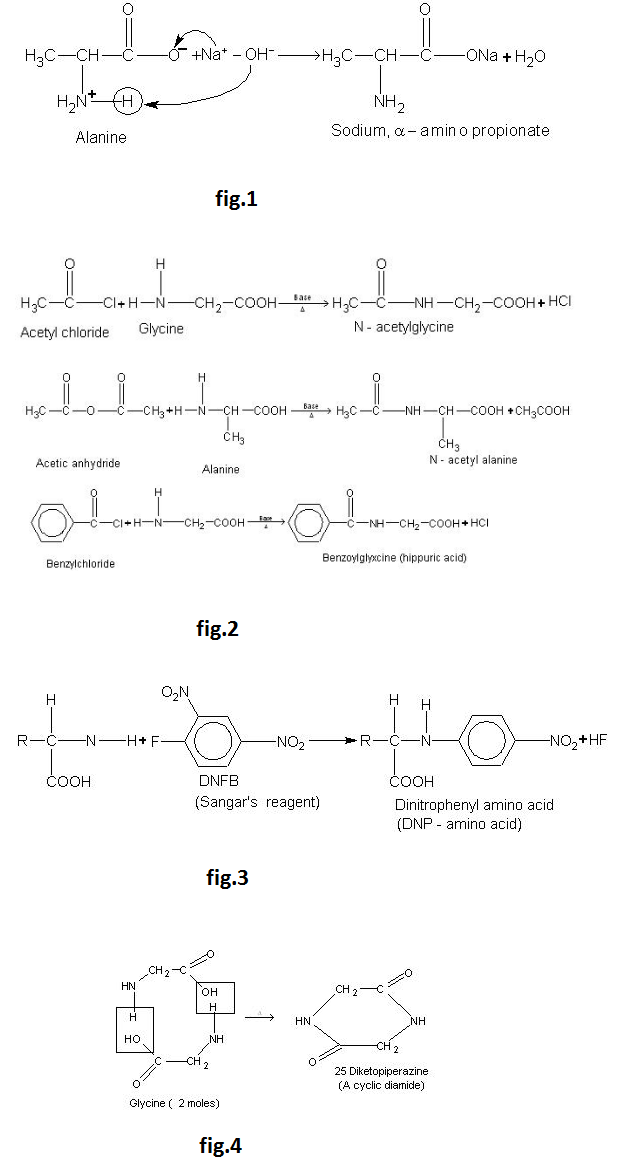
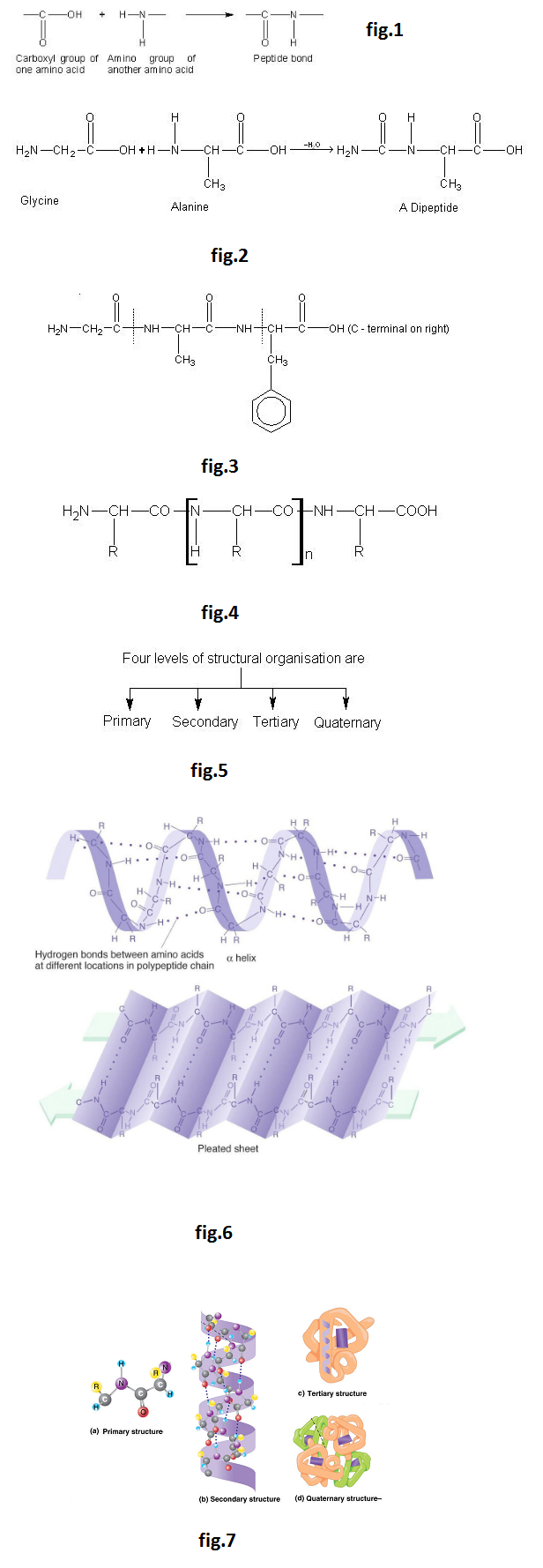
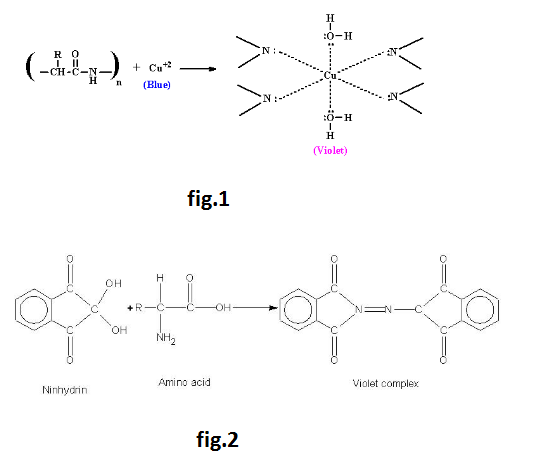
Amino Acids :
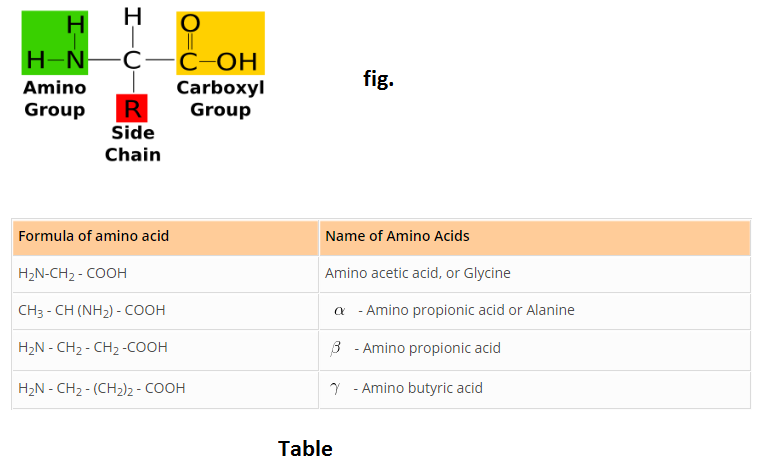
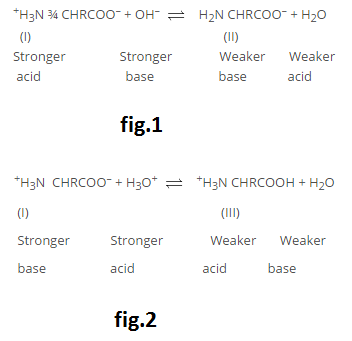
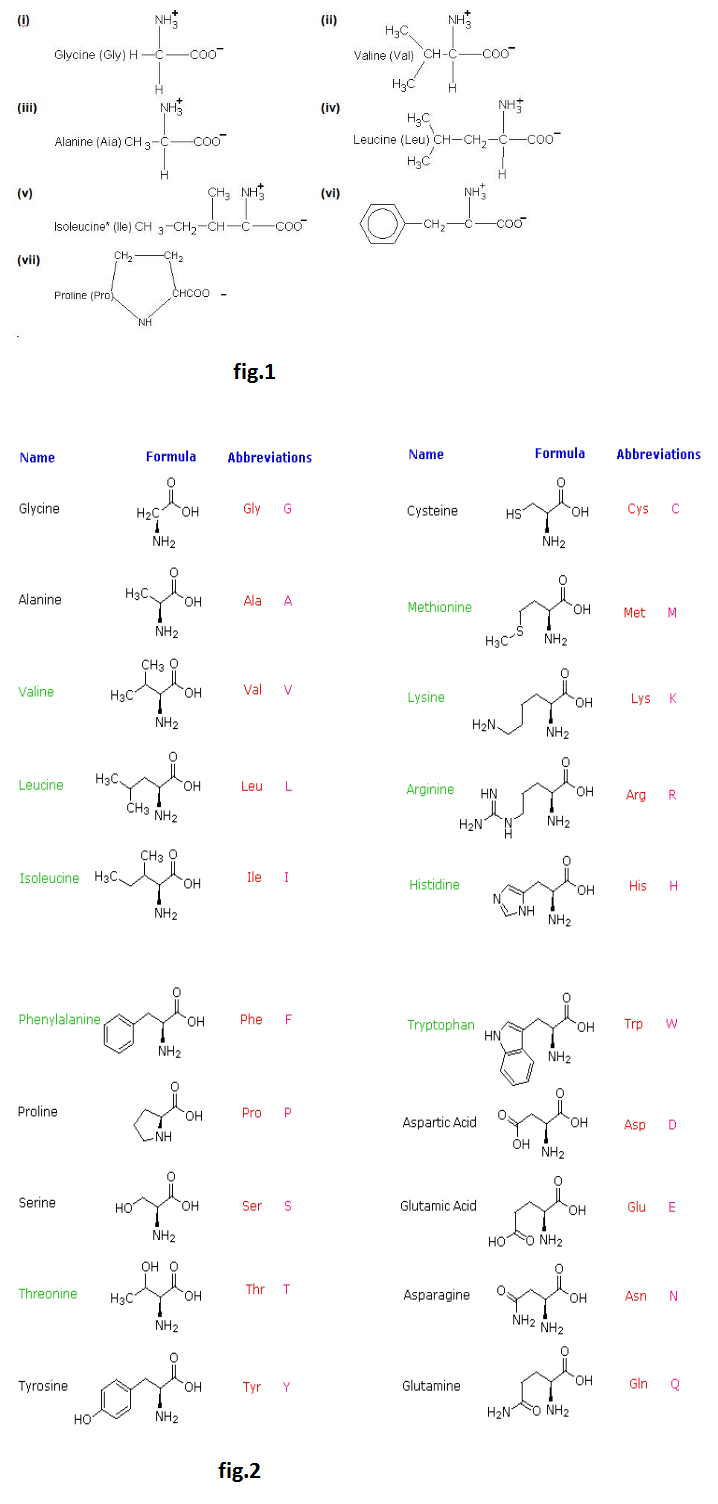
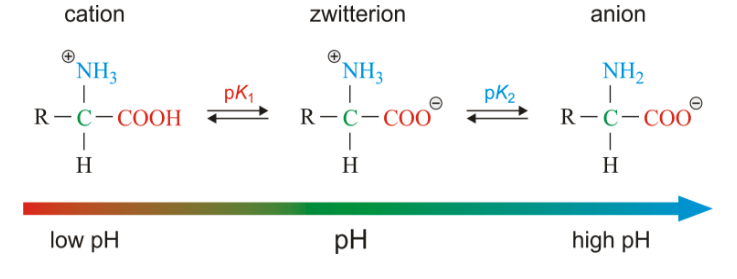
Amino acids are molecules, which contain two functional groups, one is carboxylic group and another is amino group. Amino acids are derivatives of carboxylic acids in which one hydrogen atom of carbon chain is substituted by Amino group. Amino group may be at alpha, beta or gama position with respect to carboxylic group. See fig.
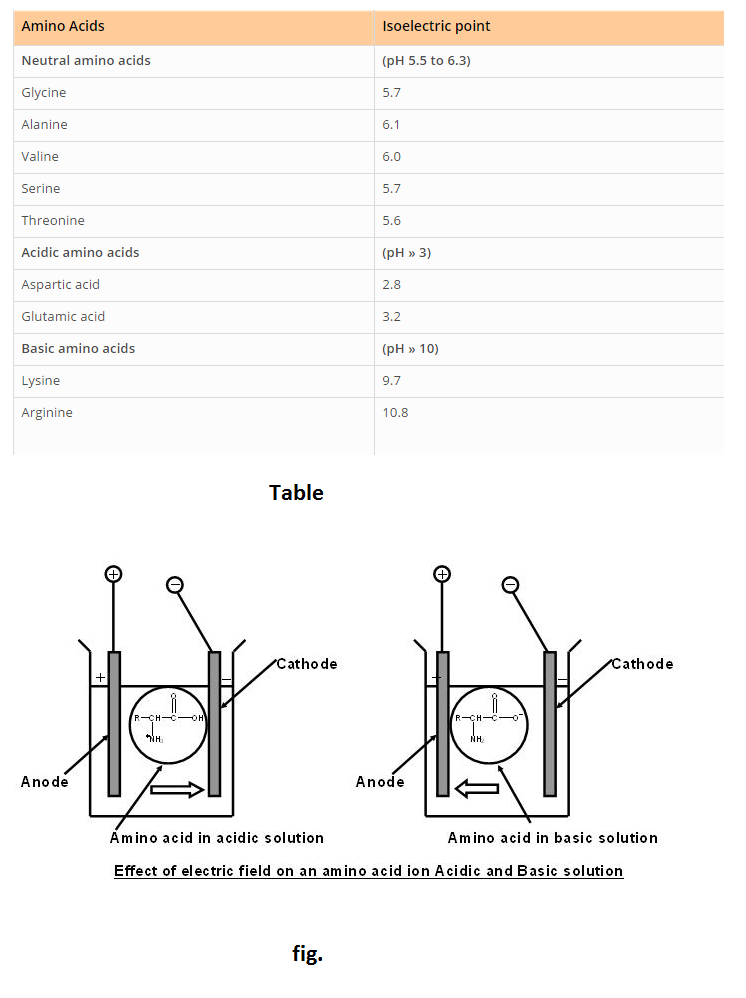
See Table.
Some amino acids contain a second carboxyl group or a potential carboxyl group in the form of carboxamide: these are called `text(acidic amino acid)` some contain a second basic group which may be an amino group these are called `text(basic amino acids.)`
See Table.
Some amino acids contain a second carboxyl group or a potential carboxyl group in the form of carboxamide: these are called `text(acidic amino acid)` some contain a second basic group which may be an amino group these are called `text(basic amino acids.)`