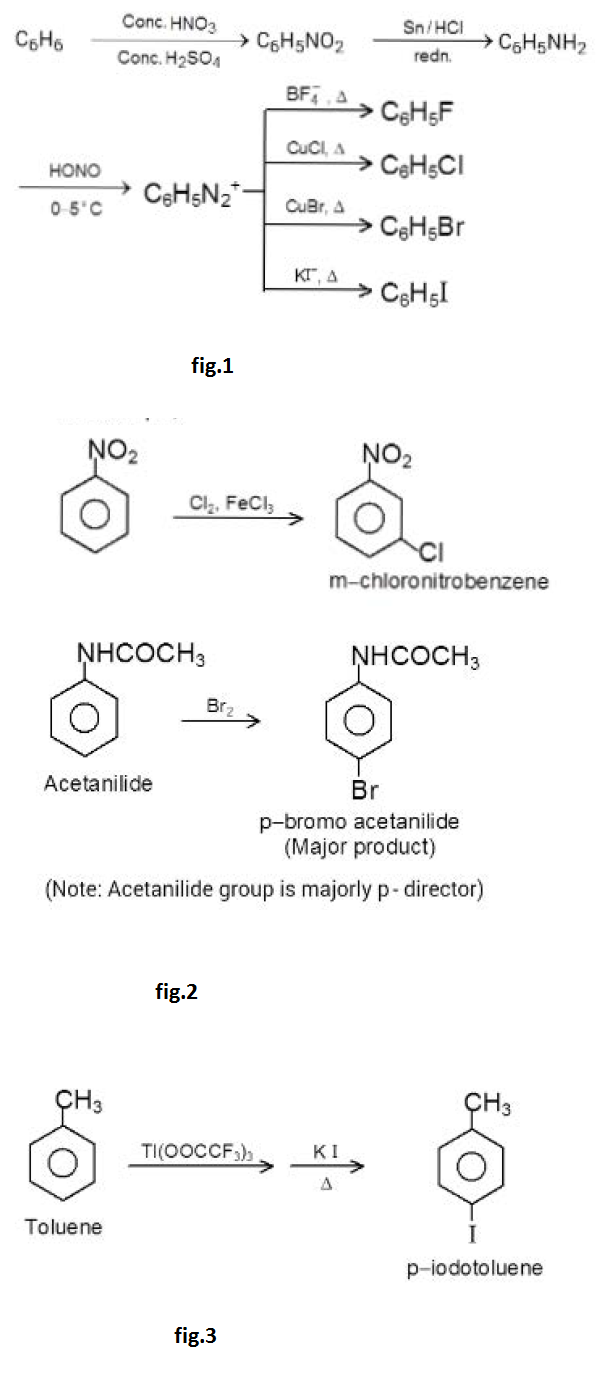
`text(LOW REACTIVITY OF ARYL AND VINYL HALIDES)`
An alkyl halide can be conveniently detected by the precipitation of insoluble silver halides when it is warmed with alcoholic `AgNO_3` . The reaction occurs instantaneously with tertiary alkyl or benzyl halides and within five minutes or so with primary and secondary halides. But halobenzene or vinyl halides can be heated with alcoholic `AgNO_3` for days without the slightest trace of silver halide being detected.
See fig.1.
The typical reaction of alkyl halides is nucleophilic substitution.
`R - X + : Z -> R-Z + : X^(-)`
where `Z = OH^(-) , OR^(-) , NH_3 , CN^(-) , NH_2^(-) , ROH , H_2O` etc
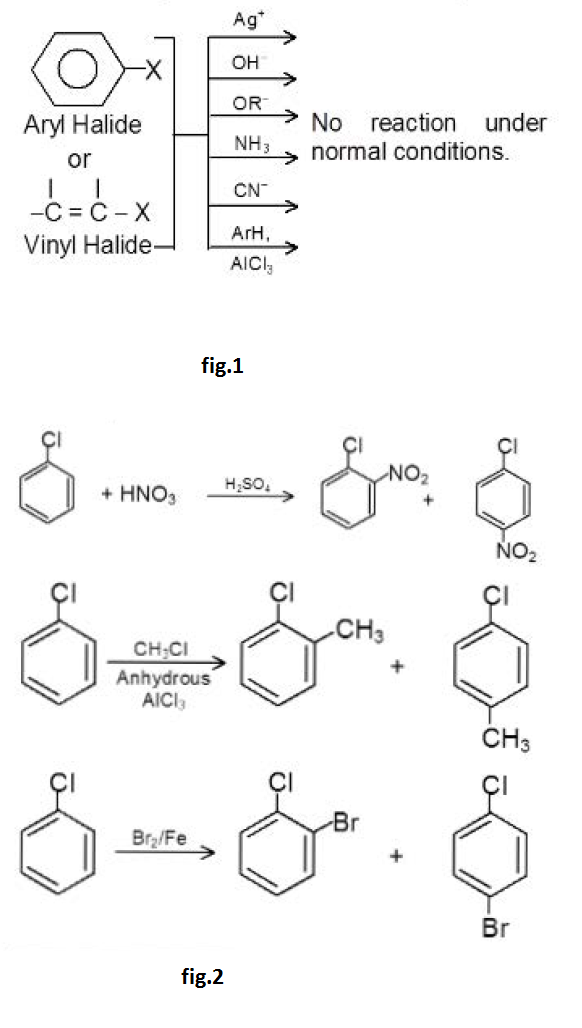
But aryl halides undergo nucleophilic substitution reactions
only in extreme conditions. Except for certain industrial
processes where very severe conditions are feasible, one
does not ordinarily prepare phenols (ArOH), ethers (ArOR),
amines `(ArNH_2)` or nitriles (ArCN) by nucleophi lic attack on
aryl halides. The aryl halides cannot be used in the FriedelCraft's
alkylation reaction just like alkyl halides.
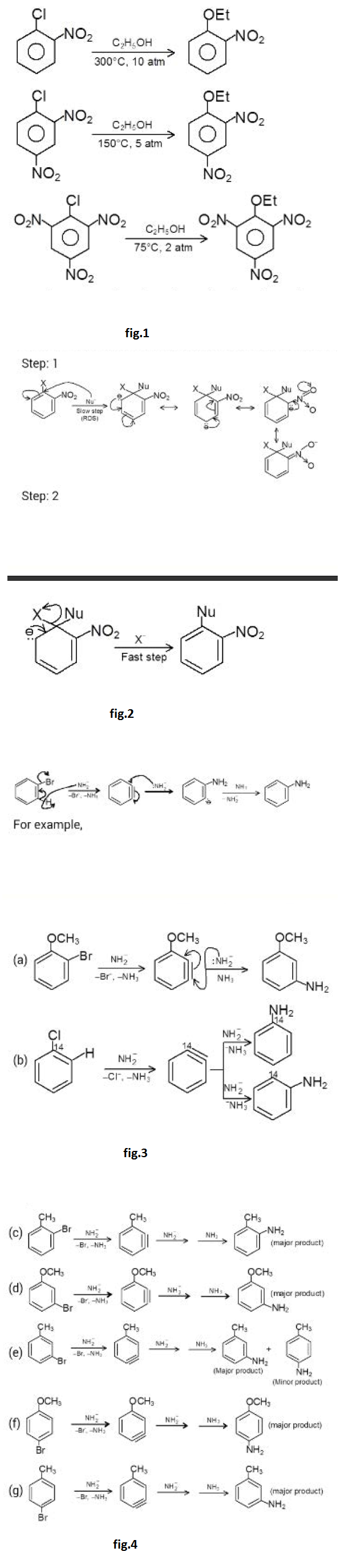
However, aryl halides do undergo nucleophilic substitution readily if the aromatic ring contains, in addition to halogen, certain other properly placed groups, which can activate the ring towards nucleophilic substitution. The presence of electron withdrawing groups like - `NO_2, - CF_3` at ortho or para position to the halogen atom makes the aryl halides more susceptible to nucleophilic attack.
The reactions of unactivated and deactivated aryl halides with strong bases or at high temperature proceed via the benzyne intermediate. The Dow's process used for the manufacture of phenol involves benzyne intermediate. Aryl halides can also undergo typical electrophilic aromatic substitution reactions like nitration. sulphonation. halogenation. Friedel - Craft's alkylation. Hal09en is unusual in being deactivating but ortho and para - directing.
`text(FORMATION OF GRIGNARD REAGENT)`
`ArBr + Mg oversettext(dry ether)-> ArMgBr`
`ArCI + Mg oversettext(tetrohydrofuran)-> ArMgCl`
`text(ELECTROPHILIC AROMATIC SUBSTITUTION)`
Although halogen is deactivating but it directs the incoming electrophile to ortho and para position.
For example,
See fig.2.
`text(LOW REACTIVITY OF ARYL AND VINYL HALIDES)`
An alkyl halide can be conveniently detected by the precipitation of insoluble silver halides when it is warmed with alcoholic `AgNO_3` . The reaction occurs instantaneously with tertiary alkyl or benzyl halides and within five minutes or so with primary and secondary halides. But halobenzene or vinyl halides can be heated with alcoholic `AgNO_3` for days without the slightest trace of silver halide being detected.
See fig.1.
The typical reaction of alkyl halides is nucleophilic substitution.
`R - X + : Z -> R-Z + : X^(-)`
where `Z = OH^(-) , OR^(-) , NH_3 , CN^(-) , NH_2^(-) , ROH , H_2O` etc
But aryl halides undergo nucleophilic substitution reactions
only in extreme conditions. Except for certain industrial
processes where very severe conditions are feasible, one
does not ordinarily prepare phenols (ArOH), ethers (ArOR),
amines `(ArNH_2)` or nitriles (ArCN) by nucleophi lic attack on
aryl halides. The aryl halides cannot be used in the FriedelCraft's
alkylation reaction just like alkyl halides.
However, aryl halides do undergo nucleophilic substitution readily if the aromatic ring contains, in addition to halogen, certain other properly placed groups, which can activate the ring towards nucleophilic substitution. The presence of electron withdrawing groups like - `NO_2, - CF_3` at ortho or para position to the halogen atom makes the aryl halides more susceptible to nucleophilic attack.
The reactions of unactivated and deactivated aryl halides with strong bases or at high temperature proceed via the benzyne intermediate. The Dow's process used for the manufacture of phenol involves benzyne intermediate. Aryl halides can also undergo typical electrophilic aromatic substitution reactions like nitration. sulphonation. halogenation. Friedel - Craft's alkylation. Hal09en is unusual in being deactivating but ortho and para - directing.
`text(FORMATION OF GRIGNARD REAGENT)`
`ArBr + Mg oversettext(dry ether)-> ArMgBr`
`ArCI + Mg oversettext(tetrohydrofuran)-> ArMgCl`
`text(ELECTROPHILIC AROMATIC SUBSTITUTION)`
Although halogen is deactivating but it directs the incoming electrophile to ortho and para position.
For example,
See fig.2.