Conductors and Non Conductors :
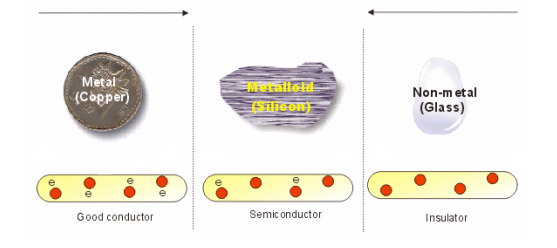
Substances around us can be divided into two classes based on their ability of conduct electricity:
• `text(Non-Conductors:)` Those substances which do not allow electric current to pass through them are called non-conductors or insulators. Example: - wood, plastic glass, rubber etc.
• `text(Conductors:)` Those substances which allow electric current to flow through them are called conductors. Examples: Copper, Iron, Gold, Silver, Graphite, salt solution etc.
Conductors can further be divided into two groups : (i) Metallic Conductors (ii) Electrolytic Conductors
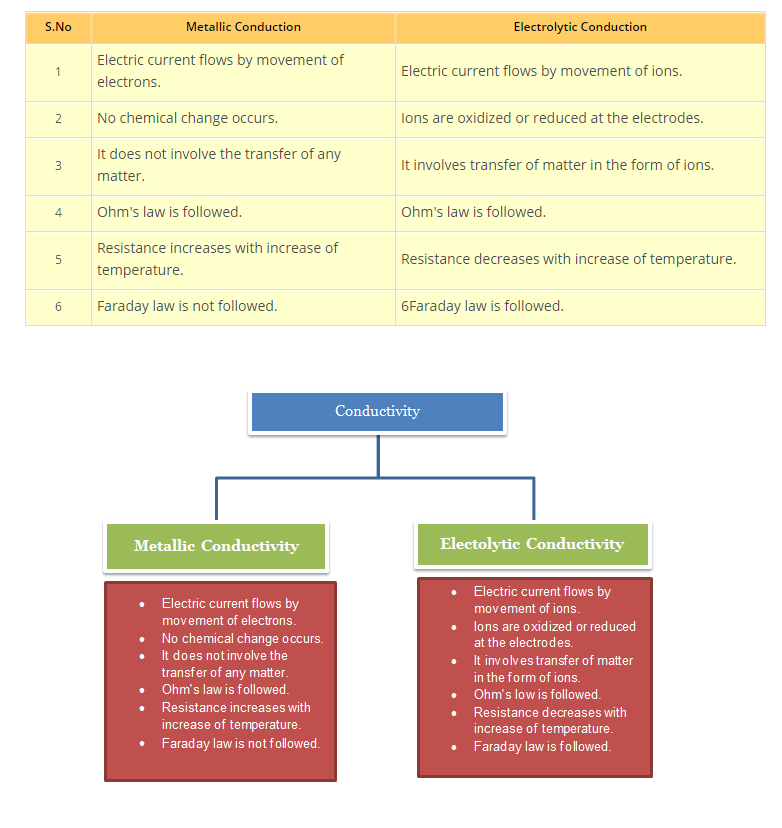
• `text(Metallic Conductors:)` These conductors conduct electricity or electric current by movement of electrons without undergoing any chemical change during the process. These conduct electricity in both solid as well as molten state. Example: All the metals and Graphite
• `text(Electrolytes:)` Those substances which conduct electricity only when they are present in aqueous solution and not in solid form are called electrolytes. These conduct electricity by movement of ions in solutions.
Non-ionic compound or covalent compounds do not conduct electricity in aqueous solution and hence they are called non-electrolytes. Examples of non- electrolytes are : Urea, Glucose, Sugar etc.
Electrolytes can further be divided into strong and weak electrolytes :
• `text(Strong Electrolytes)` are those electrolytes which dissociate completely in aqueous solution to give constituent ions. For example : Inorganic salts like `NaCl`, `KCl`, Strong Acid like `HCl`, `H_2SO_4`, Strong bases like `NaOH`, `KOH` etc.
• `text(Weak Electrolytes)` are those electrolytes which partially dissociate in aqueous solution to give constituent ions. For example : weak acid like `CH_3COOH` and Weak bases like `NH_3`.
`text(Comparison of Electrolytic and Metallic Conduction)` : See fig.
• `text(Non-Conductors:)` Those substances which do not allow electric current to pass through them are called non-conductors or insulators. Example: - wood, plastic glass, rubber etc.
• `text(Conductors:)` Those substances which allow electric current to flow through them are called conductors. Examples: Copper, Iron, Gold, Silver, Graphite, salt solution etc.
Conductors can further be divided into two groups : (i) Metallic Conductors (ii) Electrolytic Conductors
• `text(Metallic Conductors:)` These conductors conduct electricity or electric current by movement of electrons without undergoing any chemical change during the process. These conduct electricity in both solid as well as molten state. Example: All the metals and Graphite
• `text(Electrolytes:)` Those substances which conduct electricity only when they are present in aqueous solution and not in solid form are called electrolytes. These conduct electricity by movement of ions in solutions.
Non-ionic compound or covalent compounds do not conduct electricity in aqueous solution and hence they are called non-electrolytes. Examples of non- electrolytes are : Urea, Glucose, Sugar etc.
Electrolytes can further be divided into strong and weak electrolytes :
• `text(Strong Electrolytes)` are those electrolytes which dissociate completely in aqueous solution to give constituent ions. For example : Inorganic salts like `NaCl`, `KCl`, Strong Acid like `HCl`, `H_2SO_4`, Strong bases like `NaOH`, `KOH` etc.
• `text(Weak Electrolytes)` are those electrolytes which partially dissociate in aqueous solution to give constituent ions. For example : weak acid like `CH_3COOH` and Weak bases like `NH_3`.
`text(Comparison of Electrolytic and Metallic Conduction)` : See fig.