Arrhenius Theory of Electrolytic dissociation :
In order to explain the properties of electrolytic solutions, Arrhenius put forth, in 1884, a comprehensive theory which is known as theory of electrolytic dissociation or ionic theory.
`text(The main points of the theory are:)`
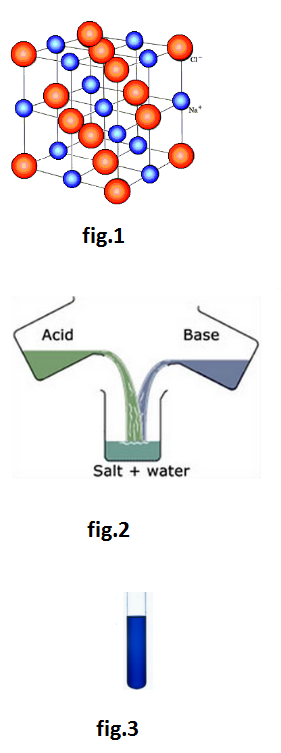
•An electrolyte, when dissolved in water, breaks up into two types of charged particles, one carrying a positive charge and the other a negative charge. These charged particles are called ions. Positively charged ions are termed cations and negatively charged as anions.
`AB -> A^(+) +B^(-)`
`NaCl -> Na^(+) +Cl^(-)`
`undersettext(Elecytrolyte)(K_2SO_4) -> undersettext(Ions)(2K^(+) +SO_4^(2-))`
In its modern form, the theory assumes that solid electrolytes are composed of ions which are held together by electrostatic forces of attraction. When an electrolyte is dissolved in a solvent, these forces are weakened and the electrolyte undergoes dissociation into ions. The ions are solvated.
•The process of splitting of the molecules into ions of an electrolyte is called ionization. The fraction of the total number of molecules present in solution as ions is known as degree of ionization or degree of dissociation. It is denoted by
`alpha = (text(Number of molecules dissociated into ions))/(text(Total number of molecules))`
•It has been observed that all electrolytes do not ionize to the same extent.
Some are almost completely ionized while others are feebly ionized. The degree of ionization depends on a number of factors.
•Ions present in solution constantly re-unite to form neutral molecules and, thus, there is a state of dynamic equilibrium between the ionized the ionized and non-ionised molecules,
i.e., `AB ⇋ A^(+) + B^(-)`
Applying the law of mass action to above equilibrium `[A^(+) ][B^(-) ] //[AB] = K`
K is known as ionization constant. The electrolytes having high value of K are termed strong electrolytes and those having low value of K as weak electrolytes.
•When an electric current is passed through the electrolytic solution, the positive ions (cations) move towards cathode and the negative ions (anions) move towards anode and get discharged, i.e., electrolysis occurs.
The ions are discharged always in equivalent amounts, no matter what their relative speeds are.
•The electrolytic solutions is always neutral in nature as the total charge on one set of ions is always equal to the total charge on the other set of ions. However, it is not necessary that the number of two sets of ions must be equal always. See Table.
•The properties of electrolytes in solution are the properties of ions present in solution. For example, acidic solution always contains `H^(+)` ions while basic solution contains `OH^(-)` ions and characteristic properties of solutions are those of `H^(-)` ions and `OH^(-)` ions respectively.
•The ions act like molecules towards depressing the freezing point, elevating the boiling point, lowering the vapour pressure and establishing the osmotic pressure.
•The conductively of the electrolytic solution depends on the nature and number of ions as the current is carried through solution by the movement of ions.
`text(The main points of the theory are:)`
•An electrolyte, when dissolved in water, breaks up into two types of charged particles, one carrying a positive charge and the other a negative charge. These charged particles are called ions. Positively charged ions are termed cations and negatively charged as anions.
`AB -> A^(+) +B^(-)`
`NaCl -> Na^(+) +Cl^(-)`
`undersettext(Elecytrolyte)(K_2SO_4) -> undersettext(Ions)(2K^(+) +SO_4^(2-))`
In its modern form, the theory assumes that solid electrolytes are composed of ions which are held together by electrostatic forces of attraction. When an electrolyte is dissolved in a solvent, these forces are weakened and the electrolyte undergoes dissociation into ions. The ions are solvated.
•The process of splitting of the molecules into ions of an electrolyte is called ionization. The fraction of the total number of molecules present in solution as ions is known as degree of ionization or degree of dissociation. It is denoted by
`alpha = (text(Number of molecules dissociated into ions))/(text(Total number of molecules))`
•It has been observed that all electrolytes do not ionize to the same extent.
Some are almost completely ionized while others are feebly ionized. The degree of ionization depends on a number of factors.
•Ions present in solution constantly re-unite to form neutral molecules and, thus, there is a state of dynamic equilibrium between the ionized the ionized and non-ionised molecules,
i.e., `AB ⇋ A^(+) + B^(-)`
Applying the law of mass action to above equilibrium `[A^(+) ][B^(-) ] //[AB] = K`
K is known as ionization constant. The electrolytes having high value of K are termed strong electrolytes and those having low value of K as weak electrolytes.
•When an electric current is passed through the electrolytic solution, the positive ions (cations) move towards cathode and the negative ions (anions) move towards anode and get discharged, i.e., electrolysis occurs.
The ions are discharged always in equivalent amounts, no matter what their relative speeds are.
•The electrolytic solutions is always neutral in nature as the total charge on one set of ions is always equal to the total charge on the other set of ions. However, it is not necessary that the number of two sets of ions must be equal always. See Table.
•The properties of electrolytes in solution are the properties of ions present in solution. For example, acidic solution always contains `H^(+)` ions while basic solution contains `OH^(-)` ions and characteristic properties of solutions are those of `H^(-)` ions and `OH^(-)` ions respectively.
•The ions act like molecules towards depressing the freezing point, elevating the boiling point, lowering the vapour pressure and establishing the osmotic pressure.
•The conductively of the electrolytic solution depends on the nature and number of ions as the current is carried through solution by the movement of ions.