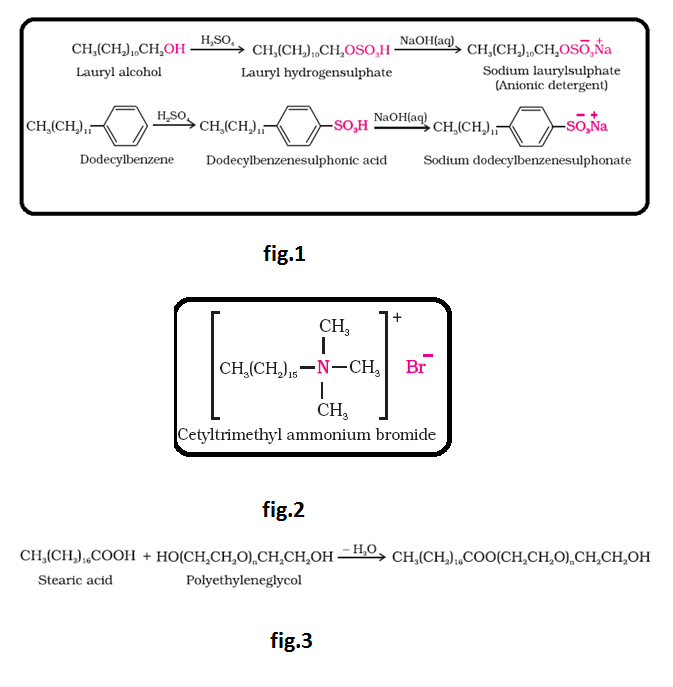
Soaps :
Sodium or potassium salts of fatty acids.
See fig.
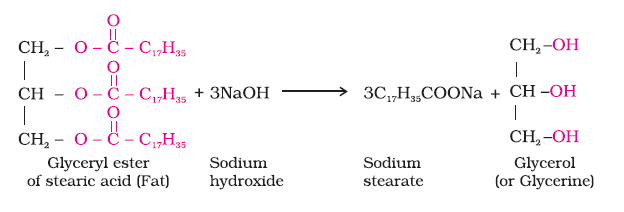
Soaps do not work with hard water as it forms insoluble salts with calcium and magnesium ions present in hard water.
See fig.
Soaps do not work with hard water as it forms insoluble salts with calcium and magnesium ions present in hard water.