`text(Reactivity of metals:)`
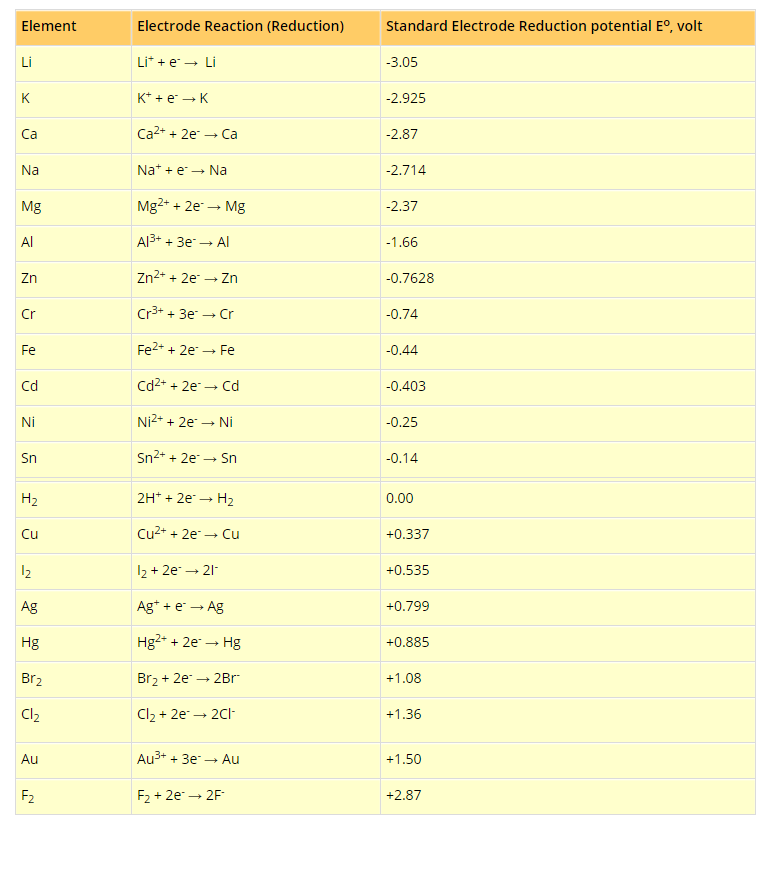
The activity of the metal depends on its tendency to lose electron or electrons, i.e., tendency to form cation (M"+). This tendency depends on the magnitude of standard reduction potential.
The metal which has high negative value (or smaller positive value) of standard reduction potential readily loses the electron or electrons and is converted into cation. Such a metal is said to be chemically active.
The chemical reactivity of metals decreases from top to bottom in the series. The metal higher in the series is more active than the metal lower in the series. For example,
`*` Alkali metals and alkaline earth metals having high negative values of standard reduction potentials are chemically active. These react with cold water and evolve hydrogen. These readily dissolve in acids forming corresponding salts and combine with those substances which accept electrons.
`*` Metals like `Fe`, `Pb`, `Sn`, `Ni`, `Co`, etc., which lie a little down in the series do not react with cold water but react with steam to evolve hydrogen.
`*` Metals like `Cu`, `Ag` and `Au` which lie below hydrogen are less reactive and do not evolve hydrogen from water.
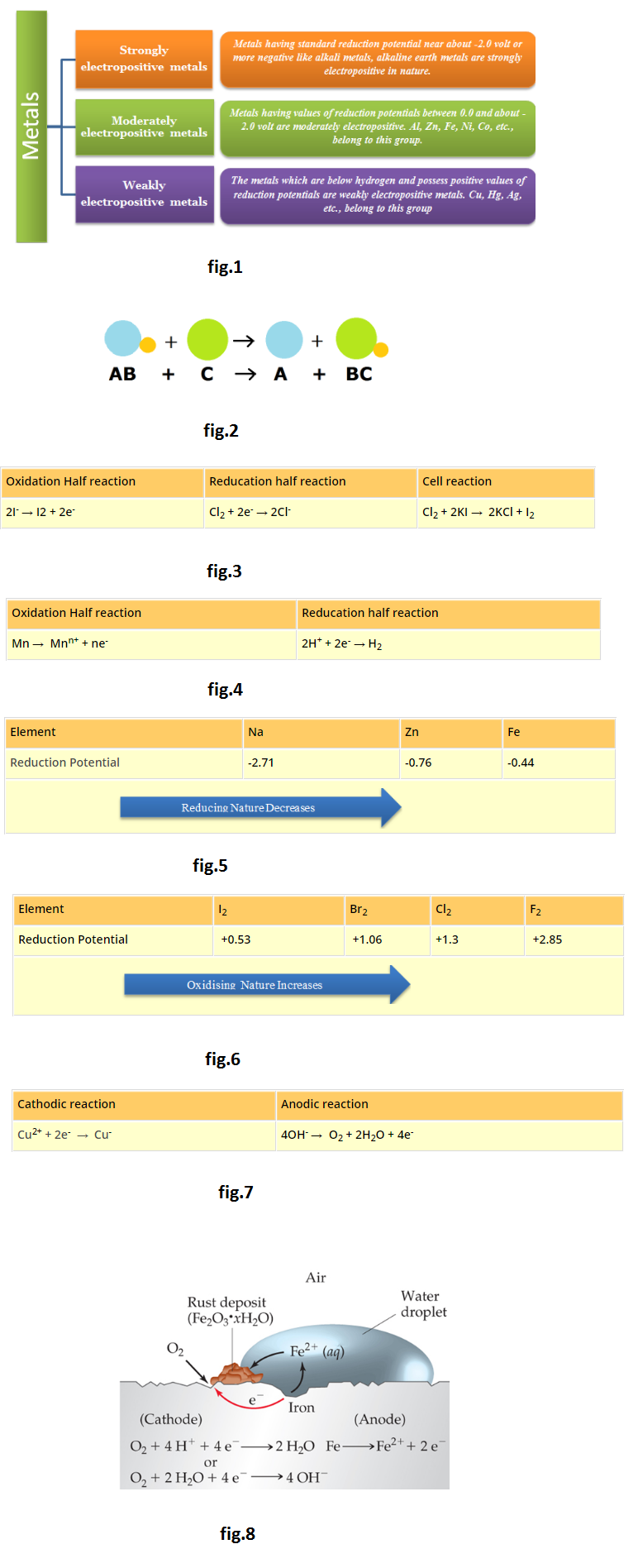
`text(Electropositive character of metals:)`
The electropositive character also depends on the tendency to lose electron or electrons. Like reactivity, the electropositive character of metals decreases from top to bottom in the electrochemical series. On the basis of standard reduction potential values, metals are divided into three groups:
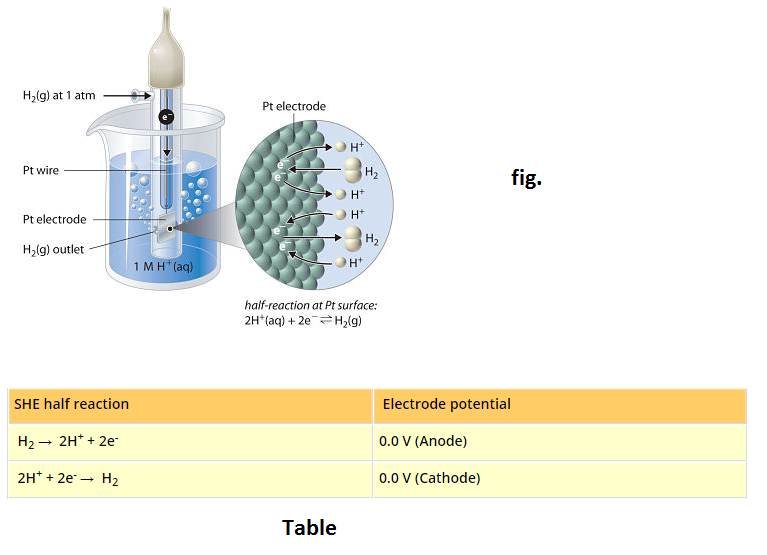
See fig.1.
`*` Strongly electropositive metals: Metals having standard reduction potential near about -2.0 volt or more negative like alkali metals, alkaline earth metals are strongly electropositive in nature.
`*` Moderately electropositive metals: Metals having values of reduction potentials between 0.0 and about -2.0 volt are moderately electropositive. `Al`, `Zn`, `Fe`, `Ni`, `Co`, etc., belong to this group.
`*` Weakly electropositive metals: The metals which are below hydrogen and possess positive values of reduction potentials are weakly electropositive metals. `Cu`, `Hg`, `Ag`, etc., belong to this group.
`text(Displacement reactions :)`
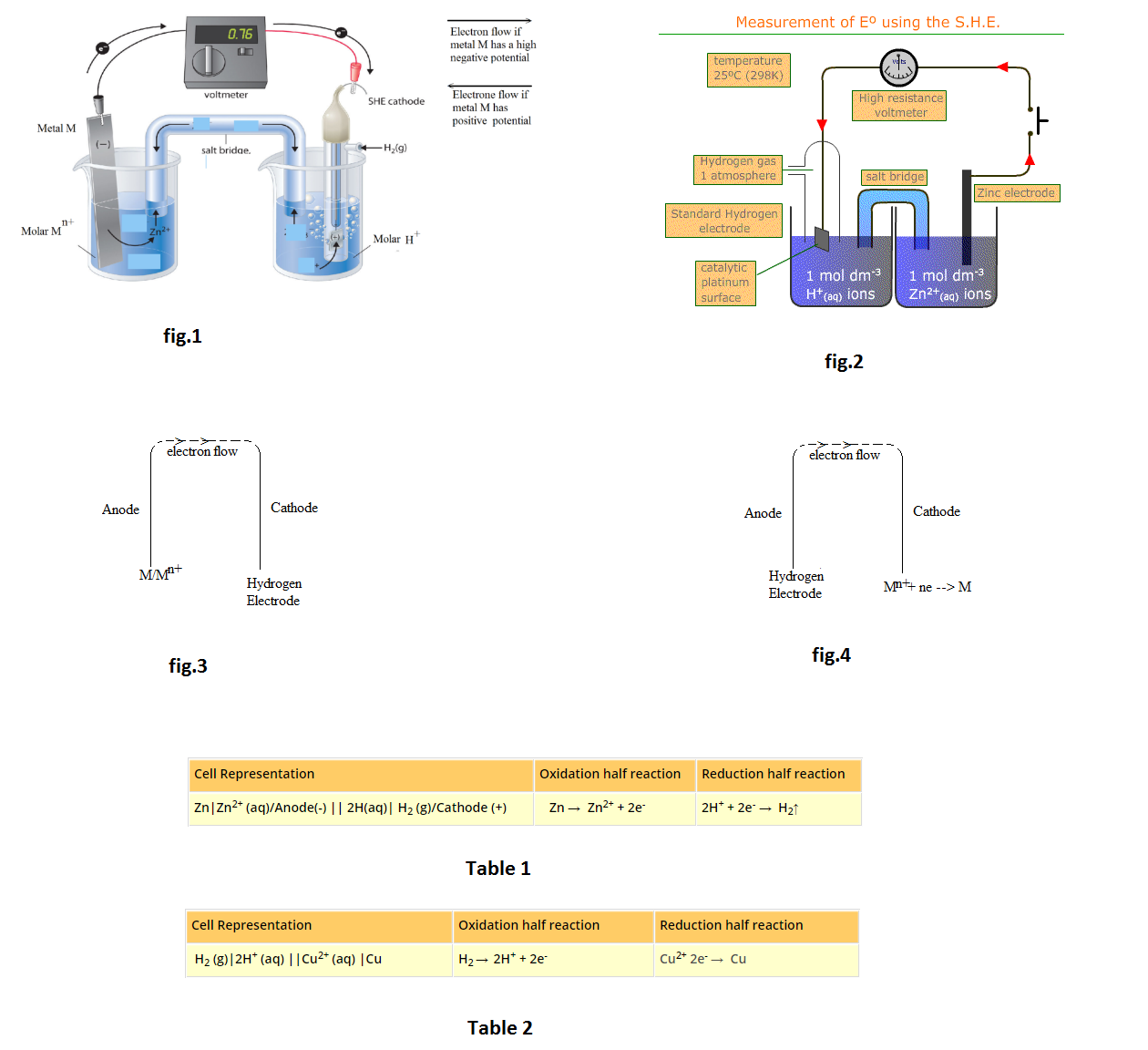
See fig.2.
`*` To predict whether a given metal will displace another, from its salt solution: A metal higher in the series will displace the metal from its solution which is lower in the series, i.e., the metal having low standard reduction potential will displace the metal from its salt's solution which has higher value of standard reduction potential. A metal higher in the series has greater tendency to provide electrons to the cations of the metal to be precipitated.
`*` Displacement of one nonmetal from its salt solution by another nonmetal: A nonmetal higher in the series (towards bottom side), i.e., having high value of reduction potential will displace another nonmetal with lower reduction potential i.e., occupying position above in the series. The nonmetal's which possess high positive reduction potentials have the tendency to accept electrons readily. These electrons are provided by the ions of the nonmetal having low value of reduction potential. Thus, `Cl_2` can displace bromine and iodine from bromides and iodides.
See fig.3.
[The activity or electronegative character or oxidising nature of the nonmetal increases as the value of reduction potential increases.]
`*` Displacement of hydrogen from dilute acids by metals: The metal which can provide electrons to H+ ions present in dilute acids for reduction, evolve hydrogen from dilute acids.
See fig.4.
The metal having negative values of reduction potential possess the property of losing electron or electrons.
Thus, the metals occupying top positions in the electrochemical series readily liberate hydrogen from dilute acids and on descending in the series tendency to liberate hydrogen gas from dilute acids decreases.
The metals which are below hydrogen in electrochemical series like Cu, Hg, Au, Pt, etc., do not evolve hydrogen from dilute acids.
`*` Displacement of hydrogen from water: Iron and the metals above iron are capable of liberating hydrogen from water. The tendency decreases from top to bottom in electrochemical series.Alkali and alkaline earth metals liberate hydrogen from cold water but Mg, Zn and Fe liberate hydrogen from hot water or steam.
`text(Reducing power of metals:)`
Reducing nature depends on the tendency of losing electron or electrons. More the negative reduction potential, more is the tendency to lose electron or electrons. Thus, reducing nature decreases from top to bottom in the electrochemical series. The power of the reducing agent increases as the standard reduction potential becomes more and more negative.
Sodium is a stronger reducing agent than zinc and zinc is a stronger reducing agent than iron.
See fig.5.
Alkali and alkaline earth metals are strong reducing agents.
Oxidising nature of nonmetals:
Oxidising nature depends on the tendency to accept electron or electrons. More the value of reduction potential, higher is the tendency to accept electron or electrons. Thus, oxidising nature increases from top to bottom in the electrochemical series. The strength of an oxidising agent increases as the value of reduction potential becomes more and more positive.
`F_2` (Fluorine) is a stronger oxidant than `Cl_2, Br_2` and `I_2`.
`Cl_2` (Chlorine) is a stronger oxidant than `Br_2` and `I_2` .
See fig.6.
`text(Thermal stability of metallic oxides:)`
`*` The thermal stability of the metal oxide depends on its electropositive nature.
`*` As the electropositivity decreases from top to bottom, the thermal stability of the oxide also decreases from top to bottom.
`*` The oxides of metals having high positive reduction potentials are not stable towards heat.
`*` The metals which come below copper form unstable oxides, i.e., these are decomposed on heating.
`Ag_2O oversettext(Heat)-> 2 Ag + O_2`
`2HgO oversettext(Heat)-> 1/2 O_2 + 2Hg`
`text(Products of electrolysis:)`
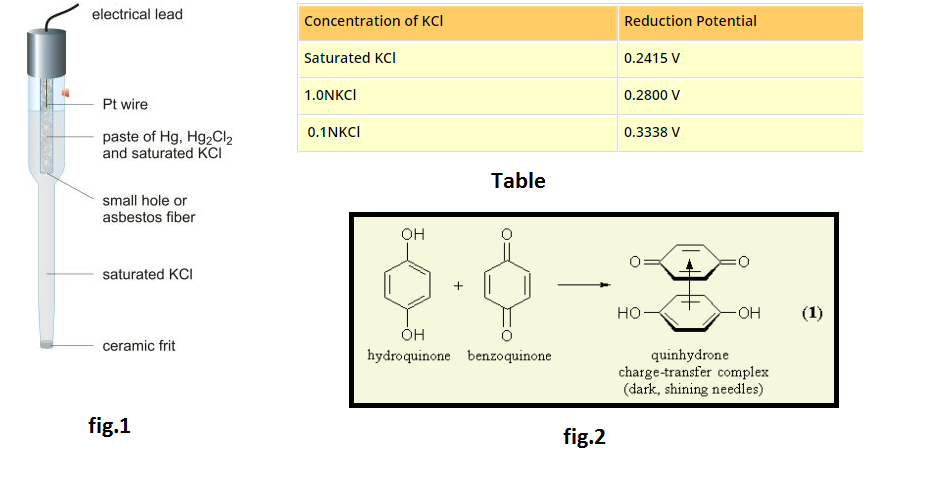
In case two or more types of positive and negative ions are present in solution, during electrolysis certain ions are discharged or liberated at the electrodes in preference to others. In general, in such competition the ion which is stronger oxidising agent (high value of standard reduction potential) is discharged first at the cathode.
The increasing order of deposition of few cations is:
`underset(->)(K^+, Ca^(2+), Na^+, Mg^(2+), Al^(3+), Zn^(2)+, Fe^(2+), H^+, Cu^(2+), Ag^+, Au^(3+))`
`text(Increasing order of deposition`
Similarly, the anion which is stronger reducing agent (low value of standard reduction potential) is liberated first at the anode.
The increasing order of discharge of few anions is:
`underset(->)(SO_4^(2-), NO_3^(-), OH^(-), Cl^(-), Br^(-), I^(-))`
`text(Increasing order of discharge)`
Thus, when an aqueous solution of NaCl containing `Na^(+), Cl^(-), H^(+)` and `OH"` ions is electrolysed, `H^(+)` ions are discharged at cathode and CF ions at the anode, i.e., `H_2` is liberated at cathode and chlorine at anode.
When an aqueous solution of `CuSO_4` containing `Cu^(2+), , H^+` and `OH^(-)` ions is electrolysed, `Cu^(2+)` ions are discharged at cathode and `OH^(-)` ions at the anode.
See fig.7.
Cu is deposited on cathode while `O_2` is liberated at anode.
`text(Corrosion of metals:)`
Corrosion is defined as the deterioration of a substance because of its reaction with its environment. This is also defined as the process by which metals have the tendency to go back to their combined state, i.e., reverse of extraction of metals.
Ordinary corrosion is a redox reaction by which metals are oxidised by oxygen in presence of moisture. Oxidation of metals occurs more readily at points of strain. Thus, a steel nail first corrodes at the tip and head. The end of a steel nail acts as an anode where iron is oxidised to `Fe^(2+)` ions.
`Fe → Fe^2 + 2e^(-)` (Anode reaction)
The electrons flow along the nail to areas containing impurities which act as cathodes where oxygen is reduced to hydroxyl ions.
`O_2 + 2H_2O + 4e^(-) → 4OH^(-)` (Cathode reaction)
See fig.8.
The overall reaction is
`2Fe + Oz + 2H_2O = 2Fe(OH)_2`
`Fe(OH)_2` may be dehydrated to iron oxide, `FeO`, or further oxidised to `Fe(OH)_3` and then dehydrated to iron rust, `Fe_2O_3` .
Several methods for protection of metals against corrosion have been developed. The most widely used are (i) plating the metal with a thin layer of a less easily oxidised metal (ii) allowing a protective film such as metal oxide (iii) galvanising-steel is coated with zinc (a more active metal).
`text(Extraction of metals:)`
A more electropositive metal can displace a less electropositive metal from its salt's solution. This principle is applied for the extraction of Ag and Au by cyanide process.
Silver from the solution containing sodium argento cyanide, `NaAg(CN)_2`, can be obtained by the addition of zinc as it is more electro-positive than Ag.
`2NaAg(CN)_2 + Zn → Na_2Zn(CN)_4 + 2Ag`
`text(Reactivity of metals:)`
The activity of the metal depends on its tendency to lose electron or electrons, i.e., tendency to form cation (M"+). This tendency depends on the magnitude of standard reduction potential.
The metal which has high negative value (or smaller positive value) of standard reduction potential readily loses the electron or electrons and is converted into cation. Such a metal is said to be chemically active.
The chemical reactivity of metals decreases from top to bottom in the series. The metal higher in the series is more active than the metal lower in the series. For example,
`*` Alkali metals and alkaline earth metals having high negative values of standard reduction potentials are chemically active. These react with cold water and evolve hydrogen. These readily dissolve in acids forming corresponding salts and combine with those substances which accept electrons.
`*` Metals like `Fe`, `Pb`, `Sn`, `Ni`, `Co`, etc., which lie a little down in the series do not react with cold water but react with steam to evolve hydrogen.
`*` Metals like `Cu`, `Ag` and `Au` which lie below hydrogen are less reactive and do not evolve hydrogen from water.
`text(Electropositive character of metals:)`
The electropositive character also depends on the tendency to lose electron or electrons. Like reactivity, the electropositive character of metals decreases from top to bottom in the electrochemical series. On the basis of standard reduction potential values, metals are divided into three groups:
See fig.1.
`*` Strongly electropositive metals: Metals having standard reduction potential near about -2.0 volt or more negative like alkali metals, alkaline earth metals are strongly electropositive in nature.
`*` Moderately electropositive metals: Metals having values of reduction potentials between 0.0 and about -2.0 volt are moderately electropositive. `Al`, `Zn`, `Fe`, `Ni`, `Co`, etc., belong to this group.
`*` Weakly electropositive metals: The metals which are below hydrogen and possess positive values of reduction potentials are weakly electropositive metals. `Cu`, `Hg`, `Ag`, etc., belong to this group.
`text(Displacement reactions :)`
See fig.2.
`*` To predict whether a given metal will displace another, from its salt solution: A metal higher in the series will displace the metal from its solution which is lower in the series, i.e., the metal having low standard reduction potential will displace the metal from its salt's solution which has higher value of standard reduction potential. A metal higher in the series has greater tendency to provide electrons to the cations of the metal to be precipitated.
`*` Displacement of one nonmetal from its salt solution by another nonmetal: A nonmetal higher in the series (towards bottom side), i.e., having high value of reduction potential will displace another nonmetal with lower reduction potential i.e., occupying position above in the series. The nonmetal's which possess high positive reduction potentials have the tendency to accept electrons readily. These electrons are provided by the ions of the nonmetal having low value of reduction potential. Thus, `Cl_2` can displace bromine and iodine from bromides and iodides.
See fig.3.
[The activity or electronegative character or oxidising nature of the nonmetal increases as the value of reduction potential increases.]
`*` Displacement of hydrogen from dilute acids by metals: The metal which can provide electrons to H+ ions present in dilute acids for reduction, evolve hydrogen from dilute acids.
See fig.4.
The metal having negative values of reduction potential possess the property of losing electron or electrons.
Thus, the metals occupying top positions in the electrochemical series readily liberate hydrogen from dilute acids and on descending in the series tendency to liberate hydrogen gas from dilute acids decreases.
The metals which are below hydrogen in electrochemical series like Cu, Hg, Au, Pt, etc., do not evolve hydrogen from dilute acids.
`*` Displacement of hydrogen from water: Iron and the metals above iron are capable of liberating hydrogen from water. The tendency decreases from top to bottom in electrochemical series.Alkali and alkaline earth metals liberate hydrogen from cold water but Mg, Zn and Fe liberate hydrogen from hot water or steam.
`text(Reducing power of metals:)`
Reducing nature depends on the tendency of losing electron or electrons. More the negative reduction potential, more is the tendency to lose electron or electrons. Thus, reducing nature decreases from top to bottom in the electrochemical series. The power of the reducing agent increases as the standard reduction potential becomes more and more negative.
Sodium is a stronger reducing agent than zinc and zinc is a stronger reducing agent than iron.
See fig.5.
Alkali and alkaline earth metals are strong reducing agents.
Oxidising nature of nonmetals:
Oxidising nature depends on the tendency to accept electron or electrons. More the value of reduction potential, higher is the tendency to accept electron or electrons. Thus, oxidising nature increases from top to bottom in the electrochemical series. The strength of an oxidising agent increases as the value of reduction potential becomes more and more positive.
`F_2` (Fluorine) is a stronger oxidant than `Cl_2, Br_2` and `I_2`.
`Cl_2` (Chlorine) is a stronger oxidant than `Br_2` and `I_2` .
See fig.6.
`text(Thermal stability of metallic oxides:)`
`*` The thermal stability of the metal oxide depends on its electropositive nature.
`*` As the electropositivity decreases from top to bottom, the thermal stability of the oxide also decreases from top to bottom.
`*` The oxides of metals having high positive reduction potentials are not stable towards heat.
`*` The metals which come below copper form unstable oxides, i.e., these are decomposed on heating.
`Ag_2O oversettext(Heat)-> 2 Ag + O_2`
`2HgO oversettext(Heat)-> 1/2 O_2 + 2Hg`
`text(Products of electrolysis:)`
In case two or more types of positive and negative ions are present in solution, during electrolysis certain ions are discharged or liberated at the electrodes in preference to others. In general, in such competition the ion which is stronger oxidising agent (high value of standard reduction potential) is discharged first at the cathode.
The increasing order of deposition of few cations is:
`underset(->)(K^+, Ca^(2+), Na^+, Mg^(2+), Al^(3+), Zn^(2)+, Fe^(2+), H^+, Cu^(2+), Ag^+, Au^(3+))`
`text(Increasing order of deposition`
Similarly, the anion which is stronger reducing agent (low value of standard reduction potential) is liberated first at the anode.
The increasing order of discharge of few anions is:
`underset(->)(SO_4^(2-), NO_3^(-), OH^(-), Cl^(-), Br^(-), I^(-))`
`text(Increasing order of discharge)`
Thus, when an aqueous solution of NaCl containing `Na^(+), Cl^(-), H^(+)` and `OH"` ions is electrolysed, `H^(+)` ions are discharged at cathode and CF ions at the anode, i.e., `H_2` is liberated at cathode and chlorine at anode.
When an aqueous solution of `CuSO_4` containing `Cu^(2+), , H^+` and `OH^(-)` ions is electrolysed, `Cu^(2+)` ions are discharged at cathode and `OH^(-)` ions at the anode.
See fig.7.
Cu is deposited on cathode while `O_2` is liberated at anode.
`text(Corrosion of metals:)`
Corrosion is defined as the deterioration of a substance because of its reaction with its environment. This is also defined as the process by which metals have the tendency to go back to their combined state, i.e., reverse of extraction of metals.
Ordinary corrosion is a redox reaction by which metals are oxidised by oxygen in presence of moisture. Oxidation of metals occurs more readily at points of strain. Thus, a steel nail first corrodes at the tip and head. The end of a steel nail acts as an anode where iron is oxidised to `Fe^(2+)` ions.
`Fe → Fe^2 + 2e^(-)` (Anode reaction)
The electrons flow along the nail to areas containing impurities which act as cathodes where oxygen is reduced to hydroxyl ions.
`O_2 + 2H_2O + 4e^(-) → 4OH^(-)` (Cathode reaction)
See fig.8.
The overall reaction is
`2Fe + Oz + 2H_2O = 2Fe(OH)_2`
`Fe(OH)_2` may be dehydrated to iron oxide, `FeO`, or further oxidised to `Fe(OH)_3` and then dehydrated to iron rust, `Fe_2O_3` .
Several methods for protection of metals against corrosion have been developed. The most widely used are (i) plating the metal with a thin layer of a less easily oxidised metal (ii) allowing a protective film such as metal oxide (iii) galvanising-steel is coated with zinc (a more active metal).
`text(Extraction of metals:)`
A more electropositive metal can displace a less electropositive metal from its salt's solution. This principle is applied for the extraction of Ag and Au by cyanide process.
Silver from the solution containing sodium argento cyanide, `NaAg(CN)_2`, can be obtained by the addition of zinc as it is more electro-positive than Ag.
`2NaAg(CN)_2 + Zn → Na_2Zn(CN)_4 + 2Ag`