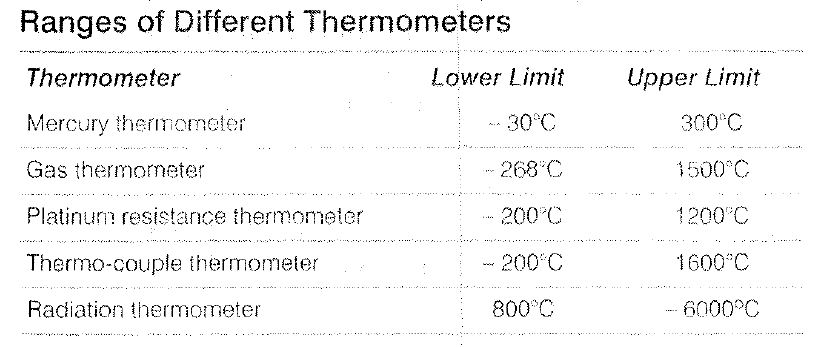
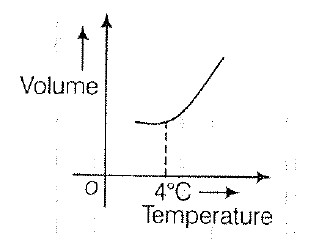
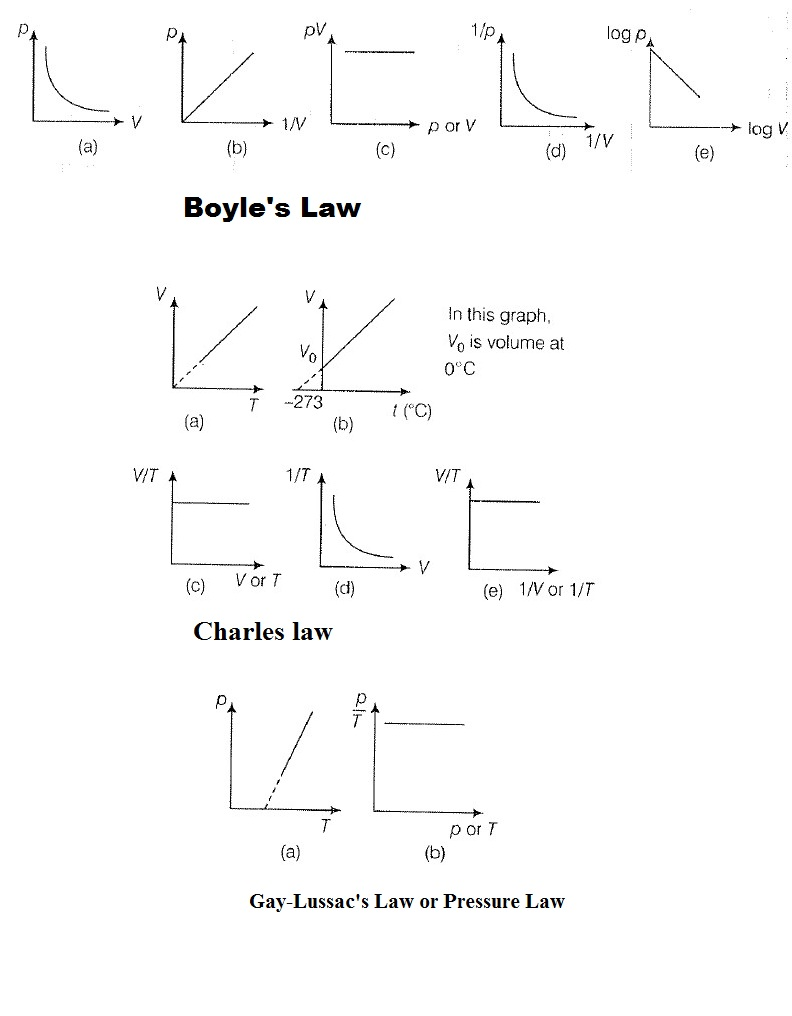
Temperature Scales
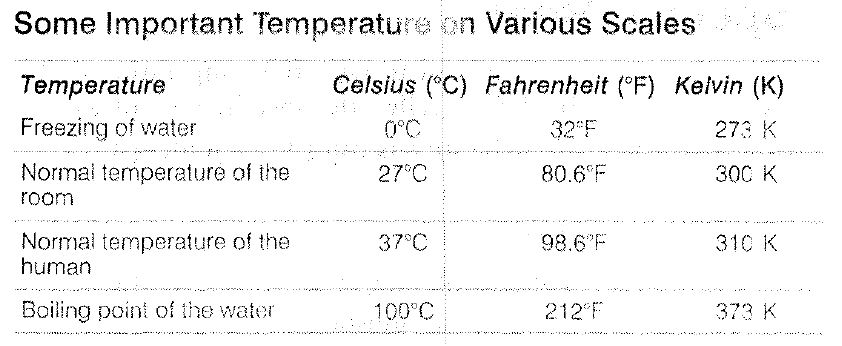
To measure temperature, two fixed points are taken; one of them is freezing point of water, known as `text(ice-point)` and other point is boiling point of water, known as `text(steam point)`.
Some temperature scales are given below
(i) `text(Celsius Scale) (°C)` In this scale of temperature, the melting point of ice is taken as 0°C and the boiling point of water as 100°C and the space between these two points is divided into 100 equal parts. This scale was designed by Anders Celsius in 1710.
(ii) `text(Fahrenheit Scale) (°F)` In this scale, the melting point of ice is taken as `32^@F` and the boiling point of water as 212°F and the space between these two points is divided into 180 equal parts. This scale was designed by Gabriel Fahrenheit in 1717.
(iii) `text(Kelvin Scale) (K)` In this scale, the ice point and the steam point (boiling point) are taken as 273 K and 373 K, respectively and the space between these two points is divided into 100 equal parts. It was designed by Kelvin.
(iv) `text(Reaumur Scale) (R)` In this scale, ice point and, boiling point are taken as `0^@R` and `80^@R` respectively. `1^@R` is equal to the 80th part of difference between two points. This scale was designed by R A Reaumur in 1730.
(v) `text(Rankine Scale) (Ra)` In this scale, ice point and steam point are taken as 460° Ra and 672° Ra, respectively. `1^@Ra` is equal to the 212th part of difference between two points.
`text(Relations between various temperature scales)`
`C/5=(F-32)/9 =(K-273)/5 = R/4 = (Ra- 460)/10.6`
Some temperature scales are given below
(i) `text(Celsius Scale) (°C)` In this scale of temperature, the melting point of ice is taken as 0°C and the boiling point of water as 100°C and the space between these two points is divided into 100 equal parts. This scale was designed by Anders Celsius in 1710.
(ii) `text(Fahrenheit Scale) (°F)` In this scale, the melting point of ice is taken as `32^@F` and the boiling point of water as 212°F and the space between these two points is divided into 180 equal parts. This scale was designed by Gabriel Fahrenheit in 1717.
(iii) `text(Kelvin Scale) (K)` In this scale, the ice point and the steam point (boiling point) are taken as 273 K and 373 K, respectively and the space between these two points is divided into 100 equal parts. It was designed by Kelvin.
(iv) `text(Reaumur Scale) (R)` In this scale, ice point and, boiling point are taken as `0^@R` and `80^@R` respectively. `1^@R` is equal to the 80th part of difference between two points. This scale was designed by R A Reaumur in 1730.
(v) `text(Rankine Scale) (Ra)` In this scale, ice point and steam point are taken as 460° Ra and 672° Ra, respectively. `1^@Ra` is equal to the 212th part of difference between two points.
`text(Relations between various temperature scales)`
`C/5=(F-32)/9 =(K-273)/5 = R/4 = (Ra- 460)/10.6`