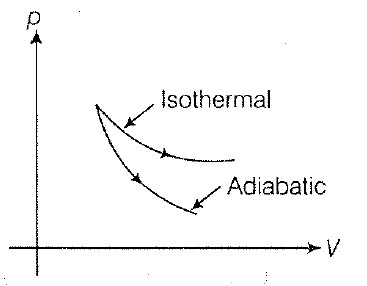
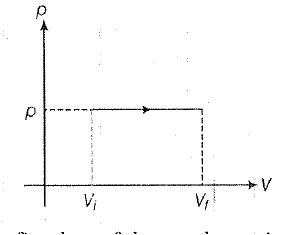
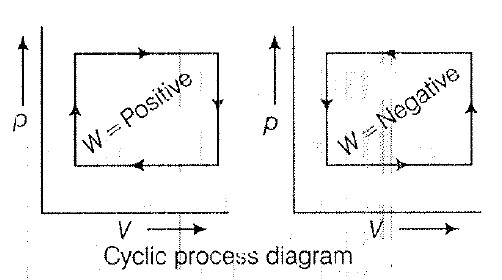
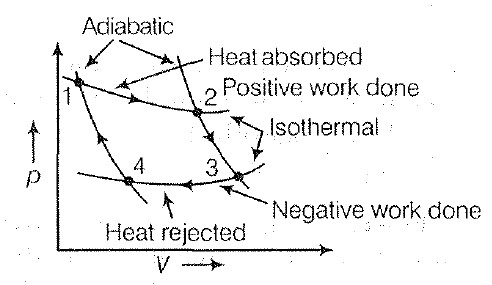
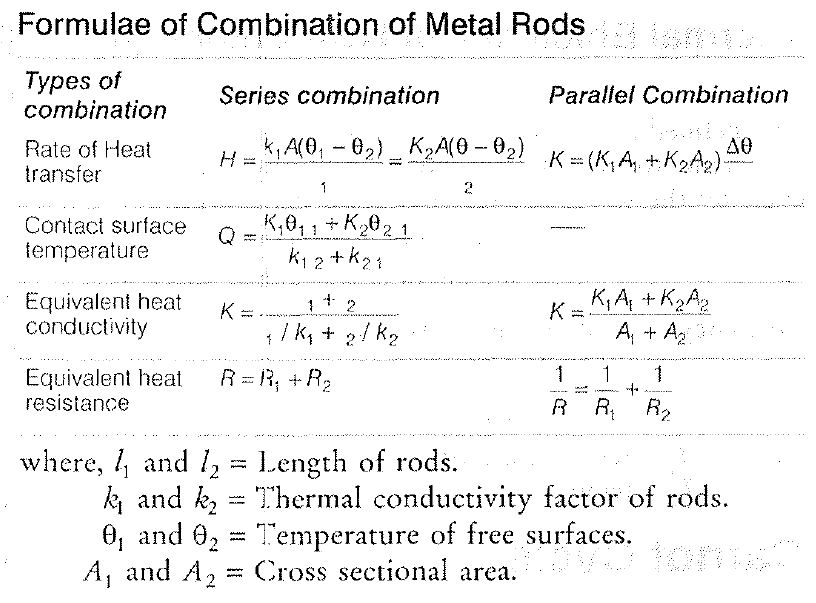
• The ability of material to conduct the heat through it, is known as thermal conductivity. Thus, heat conduction is defined as the time rate of heat flow in a material for a given temperature difference.
Consider a metal rod of length `l` and area of cross-section A. Let the ends of the rod arc at the temperatures `T_1` and `T_2`
Then, Rate of flow of heat (H) `prop` Temperature difference `Delta T`
`Hprop=` Time for which the heat flows (t)
`Hprop=` Area of cross section (A)
`Hprop=` `1/text(length of the rod)=1/L`.
Thus, the rate of heat transfer is given by
Rate of heat transfer `= (Delta Q)/(Delta t) = (KA (T_1 - T_2))/L => H = KA (Delta T)/L`
Also , `H = Q/t`
`because ` Heat transfer , `Q = K A (DeltaT)/A * t`
Here, K = coefficient of thermal conductivity of material of rod.
• The greater value of K implies that material will conduced the heat more rapidly.
• The SI unit of K is `Js^-1m^-1K^-1` or `Wm^-1K^-1`.
• The value of thermal conductivity vary slightly with temperature, but it can be considered to be constant over normal temperature range.
• If `A = 1, T_1 - T_2 = Delta T = 1, L = 1` and t = 1, then `Q = K`
Hence, the coefficient of thermal conductivity of a material may be defined as the quantity of heat that flows per unit time through a unit cube of the material, when its opposite faces are kept at a temperature difference of one degree.
• The ability of material to conduct the heat through it, is known as thermal conductivity. Thus, heat conduction is defined as the time rate of heat flow in a material for a given temperature difference.
Consider a metal rod of length `l` and area of cross-section A. Let the ends of the rod arc at the temperatures `T_1` and `T_2`
Then, Rate of flow of heat (H) `prop` Temperature difference `Delta T`
`Hprop=` Time for which the heat flows (t)
`Hprop=` Area of cross section (A)
`Hprop=` `1/text(length of the rod)=1/L`.
Thus, the rate of heat transfer is given by
Rate of heat transfer `= (Delta Q)/(Delta t) = (KA (T_1 - T_2))/L => H = KA (Delta T)/L`
Also , `H = Q/t`
`because ` Heat transfer , `Q = K A (DeltaT)/A * t`
Here, K = coefficient of thermal conductivity of material of rod.
• The greater value of K implies that material will conduced the heat more rapidly.
• The SI unit of K is `Js^-1m^-1K^-1` or `Wm^-1K^-1`.
• The value of thermal conductivity vary slightly with temperature, but it can be considered to be constant over normal temperature range.
• If `A = 1, T_1 - T_2 = Delta T = 1, L = 1` and t = 1, then `Q = K`
Hence, the coefficient of thermal conductivity of a material may be defined as the quantity of heat that flows per unit time through a unit cube of the material, when its opposite faces are kept at a temperature difference of one degree.